Hoffman Clips
$ 5.57 Original price was: $ 5.57.$ 5.42Current price is: $ 5.42.
Hoffman Voltameter
$ 58.16 Original price was: $ 58.16.$ 58.05Current price is: $ 58.05.
Whatsapp Order
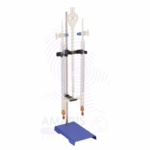
Hoffman Voltameter is a specialized laboratory apparatus used to demonstrate and measure the electrolysis of water into hydrogen and oxygen gases. It consists of a central reservoir connected to two vertical graduated gas-collection tubes (electrodes) and is typically made from borosilicate glass for chemical resistance and clarity. Platinum or carbon electrodes are used at the base of each tube to conduct electric current. This apparatus is widely used in chemistry and physics education to visually demonstrate the principles of electrolysis, electrochemical reactions, gas laws, and stoichiometry.
Description
Table of Contents
Toggle
Hoffman Voltameter
Primary Uses
- Electrochemical and Educational Applications
- Demonstrating the electrolysis of water in teaching environments.
- Measuring the volumetric ratio of hydrogen to oxygen (2:1) produced during electrolysis.
- Studying redox reactions and electrochemical cell behavior.
- Visualizing decomposition reactions and gas evolution in real time.
- Explaining Faraday’s laws of electrolysis and conservation of mass.
Secondary Uses
- Research and Analytical Chemistry
- Analyzing the purity of water or electrolyte solutions based on gas output.
- Used in historical and comparative studies of electrochemical apparatus design.
- Supporting experiments related to stoichiometry and reaction efficiency.
- Calibration and validation of electrolytic systems in academic laboratories.
KEY PRODUCT FEATURES
1.Basic Identification Attributes
- Material: Borosilicate glass body with platinum or carbon electrodes
- Design: Two graduated gas collection tubes and central electrolyte reservoir
- Components: Electrodes, stopcocks, collection tubes, supporting base
2.Physical & Chemical Properties
- Chemically resistant to acids and electrolytes
- High thermal and electrical insulation properties
- Transparent for easy observation of gas generation
3.Safety & Hazard Attributes
- Fragile glass components; must be handled carefully
- Electrolytic process produces flammable hydrogen gas—requires ventilation
- Risk of mild electric shock if mishandled during operation
4.Storage & Handling Attributes
- Store upright in a padded container to avoid breakage
- Rinse and dry completely after use to prevent corrosion or contamination
- Handle with care, especially during setup and disassembly
5.Regulatory & Compliance Attributes
- Compliant with educational laboratory standards
- Suitable for use in classrooms, colleges, and controlled laboratory environments
6.Environmental & Health Impact
- Reusable and long-lasting; no significant environmental footprint
- Produces hydrogen and oxygen gas with no toxic by-products
SAFETY HANDLING PRECAUTIONS
Safety Handling Precautions
- Use in a well-ventilated area to avoid gas buildup
- Wear protective gloves and goggles when operating
- Disconnect power before handling or cleaning
First Aid Measures
- For minor electric shock, disconnect power and seek medical attention if symptoms persist
- In case of glass breakage, treat cuts and dispose of shards safely
Firefighting Measures
- The apparatus itself is non-flammable
- Hydrogen gas produced is flammable; use CO₂ or foam extinguishers for surrounding fires
Related products
Aspirator Bottle Glass
Aspirator Bottle Glass is a durable, high-quality glass container designed specifically for use in laboratory suction and aspiration systems. These bottles are engineered to safely collect and contain liquids and aerosols during filtration, vacuum, or aspiration procedures. Made from chemically resistant glass, Aspirator Bottles offer excellent clarity and durability while withstanding the rigors of laboratory use. They typically feature secure screw caps or stopper closures that ensure leak-proof performance and easy handling. Widely used in medical, research, and industrial laboratories, Aspirator Bottle Glass is essential for safe fluid management in various applications.
conical flask
Conical flasks, also known as Erlenmeyer flasks, are widely used laboratory glassware characterized by a flat bottom, conical body, and a narrow neck. Made typically from borosilicate glass, they are designed to hold, mix, and heat liquids safely. The narrow neck helps reduce evaporation and splashing during experiments. Conical flasks are essential for titrations, culturing microorganisms, and general solution preparation in laboratories and industrial settings.
Distillation Apparatus
Distillation Apparatus is a laboratory setup designed for the separation, purification, and collection of liquid mixtures based on differences in boiling points. It typically consists of components such as a distillation flask, condenser, receiving flask, and connecting tubes, often made from chemically resistant borosilicate glass. This apparatus allows controlled heating, vaporization, condensation, and collection of volatile substances. Widely used in chemical laboratories, pharmaceutical manufacturing, food and beverage processing, and research, the distillation apparatus is essential for isolating pure liquids, removing impurities, and analyzing complex mixtures.
Dropper
Dropper is a small laboratory tool used to transfer precise volumes of liquids in drops. Typically composed of a glass or plastic tube with a squeezable rubber bulb at one end, the dropper allows controlled dispensing of liquids for experiments, titrations, or sample preparations. Its simple design enables easy handling and measurement of small quantities, making it indispensable in chemical, biological, pharmaceutical, and educational laboratories. Droppers are ideal for adding reagents dropwise, conducting qualitative tests, or transferring liquids without contamination.
Dropping Funnel with Tap
Dropping Funnel with Tap is a precision laboratory glassware apparatus designed to add liquids dropwise or in a controlled flow to a reaction vessel or system. Typically made from chemically resistant borosilicate glass, this funnel features a conical body with a ground glass joint and an integrated stopcock (tap) at the bottom for precise flow regulation. It allows gradual addition of reactants during sensitive chemical processes, minimizing splashing, sudden reactions, or contamination. Widely used in organic synthesis, titrations, and controlled mixing procedures, it ensures safety and accuracy in laboratory operations.
Gas Jar with Lid
Gas Jar with Lid is a durable, chemically resistant container designed for the collection, storage, and measurement of gases in laboratory settings. Typically made from high-quality borosilicate glass or plastic, the gas jar comes with a tight-fitting lid—often equipped with ports or holes—to ensure an airtight seal and facilitate gas transfer or measurement. This apparatus is essential for conducting gas collection experiments safely and accurately, preventing contamination and gas loss. Widely used in educational, research, and industrial laboratories, gas jars with lids enable efficient handling of gases such as oxygen, hydrogen, carbon dioxide, and other experimental gases.
Microscope mirror
A Microscope Mirror is an optical accessory used to direct and focus external light onto the specimen being examined under a microscope. Traditionally mounted beneath the microscope stage, the mirror reflects ambient or artificial light upward through the condenser and specimen to illuminate the sample. Microscope mirrors typically have one flat and one concave reflective surface, allowing users to adjust the intensity and focus of the light beam. Made from highly polished glass or metal with reflective coatings, these mirrors are essential in microscopes lacking built-in illumination systems or as backup lighting aids. They enhance visibility, contrast, and detail, making them valuable in educational, medical, and research laboratory settings.
U Tube Lab Glass With Side Arms
U Tube Lab Glass with Side Arms is a specialized U-shaped borosilicate glass apparatus featuring additional side arms for fluid or gas connections. Designed for laboratory experiments involving pressure measurement, fluid flow, and gas displacement, this device offers enhanced versatility. Made from chemically resistant, durable glass, it withstands temperature fluctuations and chemical exposure. The side arms facilitate easy integration with tubing and other equipment, enabling complex experimental setups in physics, chemistry, and engineering labs. It is widely used in manometer applications, vacuum experiments, and fluid dynamics research.


 Preservatives(food)
Preservatives(food) Flavor Enhancers
Flavor Enhancers Acidulants
Acidulants Sweeteners
Sweeteners Antioxidants
Antioxidants Colorants(food)
Colorants(food) Nutraceutical Ingredients (food)
Nutraceutical Ingredients (food) Nutrient Supplements
Nutrient Supplements Emulsifiers
Emulsifiers
 Collectors
Collectors Dust Suppressants
Dust Suppressants Explosives and Blasting Agents
Explosives and Blasting Agents Flocculants and Coagulants
Flocculants and Coagulants Frothers
Frothers Leaching Agents
Leaching Agents pH Modifiers
pH Modifiers Precious Metal Extraction Agents
Precious Metal Extraction Agents
 Antioxidants(plastic)
Antioxidants(plastic) Colorants (Pigments, Dyes)
Colorants (Pigments, Dyes) Fillers and Reinforcements
Fillers and Reinforcements Flame Retardants
Flame Retardants Monomers
Monomers Plasticizers
Plasticizers Polymerization Initiators
Polymerization Initiators Stabilizers (UV, Heat)
Stabilizers (UV, Heat)
 Antifoaming Agents
Antifoaming Agents Chelating Agents
Chelating Agents Coagulants and Flocculants
Coagulants and Flocculants Corrosion Inhibitors
Corrosion Inhibitors Disinfectants and Biocides
Disinfectants and Biocides Oxidizing Agents
Oxidizing Agents pH Adjusters
pH Adjusters Scale Inhibitors( water)
Scale Inhibitors( water)
 Antioxidants(cosmetic)
Antioxidants(cosmetic) Emollients
Emollients Fragrances and Essential Oils
Fragrances and Essential Oils Humectants
Humectants Preservatives
Preservatives Surfactants(cosmetic)
Surfactants(cosmetic) Thickeners
Thickeners UV Filters
UV Filters
 Fertilizers
Fertilizers Soil Conditioners
Soil Conditioners Plant Growth Regulators
Plant Growth Regulators Animal Feed Additives
Animal Feed Additives Biostimulants
Biostimulants Pesticides (Herbicides, Insecticides, Fungicides)
Pesticides (Herbicides, Insecticides, Fungicides)
 Active Pharmaceutical Ingredients (APIs)
Active Pharmaceutical Ingredients (APIs) Excipients
Excipients Solvents(pharmaceutical)
Solvents(pharmaceutical) Antibiotics
Antibiotics Antiseptics and Disinfectants
Antiseptics and Disinfectants Vaccine Adjuvants
Vaccine Adjuvants Nutraceutical Ingredients (pharmaceutical)
Nutraceutical Ingredients (pharmaceutical) Analgesics & Antipyretics
Analgesics & Antipyretics
 Analytical Reagents
Analytical Reagents Solvents(lab)
Solvents(lab) Chromatography Chemicals
Chromatography Chemicals Spectroscopy Reagents
Spectroscopy Reagents microbiology-and-cell-culture-reagents
microbiology-and-cell-culture-reagents Molecular Biology Reagents
Molecular Biology Reagents Biochemical Reagents
Biochemical Reagents Inorganic and Organic Standards
Inorganic and Organic Standards Laboratory Safety Chemicals
Laboratory Safety Chemicals Specialty Laboratory Chemicals(Special Laboratory Equipment)
Specialty Laboratory Chemicals(Special Laboratory Equipment)
 Demulsifiers
Demulsifiers Hydraulic Fracturing Fluids
Hydraulic Fracturing Fluids Scale Inhibitors(oil)
Scale Inhibitors(oil) Surfactants(oil)
Surfactants(oil) Drilling Fluids
Drilling Fluids
 Dyes and Pigments
Dyes and Pigments Bleaching Agents
Bleaching Agents Softening Agents
Softening Agents Finishing Agents
Finishing Agents Antistatic Agents
Antistatic Agents
 Admixtures
Admixtures Waterproofing Agents
Waterproofing Agents Sealants and Adhesives
Sealants and Adhesives Curing Compounds
Curing Compounds Concrete Repair Chemicals
Concrete Repair Chemicals Anti-Corrosion Coatings
Anti-Corrosion Coatings
 Surfactants(cleaning)
Surfactants(cleaning) Builders
Builders Enzymes
Enzymes Solvents (Cleaning)
Solvents (Cleaning) Fragrances
Fragrances
 Electronic Chemicals
Electronic Chemicals Catalysts
Catalysts Lubricants
Lubricants Photographic Chemicals
Photographic Chemicals Refrigerants
Refrigerants Automotive chemicals
Automotive chemicals Pyrotechnic Chemicals
Pyrotechnic Chemicals
 Biodegradable Surfactants
Biodegradable Surfactants Bio-based Solvents
Bio-based Solvents Renewable Polymers
Renewable Polymers Carbon Capture Chemicals
Carbon Capture Chemicals Wastewater Treatment Chemicals
Wastewater Treatment Chemicals
 Pigments
Pigments Solvents(paint)
Solvents(paint) Specialty Coatings
Specialty Coatings Binders/Resins
Binders/Resins Additives
Additives Driers
Driers Anti-Corrosion Agents
Anti-Corrosion Agents Functional Coatings
Functional Coatings Application-Specific Coatings
Application-Specific Coatings
 Leavening Agents
Leavening Agents Dough Conditioners
Dough Conditioners Flour Treatments
Flour Treatments Fat Replacers
Fat Replacers Decoratives
Decoratives Preservatives(baking)
Preservatives(baking)
 Plasticizers & Softeners
Plasticizers & Softeners Reinforcing Agents
Reinforcing Agents Adhesion Promoters
Adhesion Promoters Vulcanizing Agents
Vulcanizing Agents Antidegradants
Antidegradants Blowing Agents
Blowing Agents Fillers & Extenders
Fillers & Extenders Accelerators & Retarders
Accelerators & Retarders