Back to products


Ferric Chloride Anhydrous Extra Pure
$ 17.20 Original price was: $ 17.20.$ 17.05Current price is: $ 17.05.
Fehling Solution 1 and 2 Extra Pure
$ 35.20 Original price was: $ 35.20.$ 35.09Current price is: $ 35.09.
Whatsapp Order
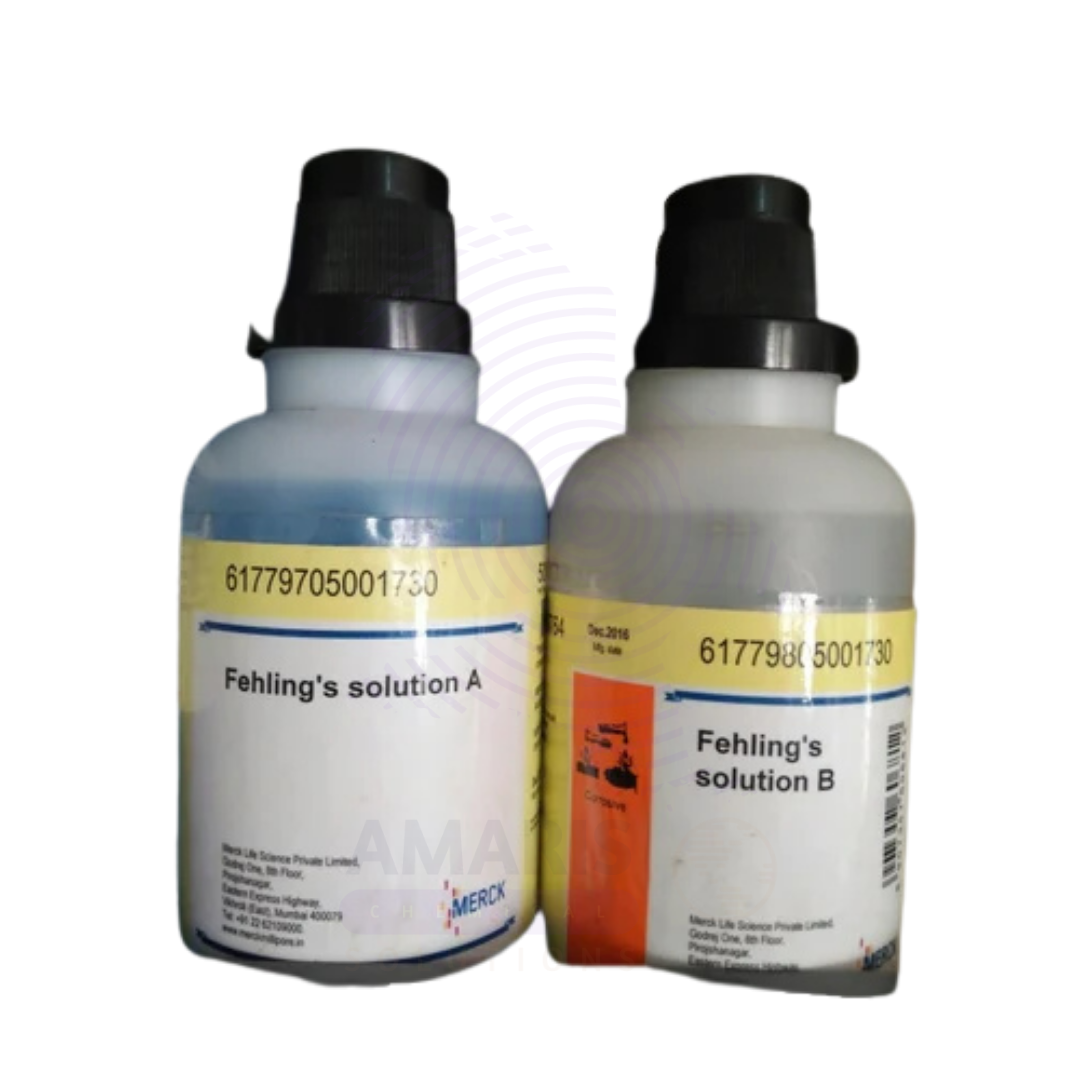
Fehling Solution 1 and 2 Extra Pure are essential reagents in classical laboratory chemistry, particularly used for the qualitative detection of reducing sugars such as glucose.
- Fehling Solution 1 contains copper(II) sulfate, while
- Fehling Solution 2 contains an alkaline potassium sodium tartrate (Rochelle salt) solution.
When mixed in equal parts just before use, they form a deep blue complex that, upon heating with a reducing sugar, yields a brick-red precipitate of cuprous oxide, confirming the presence of aldehyde groups.
These solutions are highly valuable in educational labs, food testing, and biochemical experiments. Store them separately in cool, dry conditions, and always mix fresh for accurate results.
Description
Table of Contents
Toggle
Fehling Solution 1 and 2 Extra Pure
Primary Uses
- Detection of reducing sugars:
Widely used in qualitative analysis to test for the presence of aldehyde functional groups, especially in reducing sugars like glucose. - Differentiation between aldehydes and ketones:
Reacts with aldehydes to form a red precipitate of copper(I) oxide, while ketones generally do not react. - Educational experiments in redox chemistry:
Demonstrates redox reactions and the principle of oxidation-reduction using visible color change.
Secondary Uses
- Identification of carbohydrate content in unknown samples:
Used to confirm the presence of reducing sugars in plant extracts, juices, or hydrolyzed polysaccharides. - Routine qualitative analysis in food chemistry labs:
Applied in basic food testing kits and protocols to check sugar levels in various food products. - Historical method reference in pharmaceutical analysis:
Though mostly replaced by modern techniques, still referenced in classic pharmaceutical and biochemical protocols.
Additional information
| PACK SIZE | 2.5 Litres Glass bottle |
|---|
KEY PRODUCT FEATURES
1. Basic Identification Attributes
- Chemical Name: Fehling Solution (Part I & II)
- Components:
- Fehling’s Solution I: Aqueous copper(II) sulfate (CuSO₄·5H₂O) solution
- Fehling’s Solution II: Alkaline sodium potassium tartrate (Rochelle salt) in sodium hydroxide solution
- Grade: Extra Pure (Laboratory Reagent Grade)
- Appearance:
- Solution I: Clear blue solution
- Solution II: Colorless or pale solution
2. Physical & Chemical Properties
- Fehling’s Solution I:
- Copper(II) sulfate concentration: Typically ~0.5 M
- Fehling’s Solution II:
- Sodium potassium tartrate: Chelates the copper ions
- Sodium hydroxide concentration: ~1.5 M
- When mixed (equal volumes of I & II): Deep blue complex due to formation of a copper-tartrate complex
3. Safety & Hazard Attributes
- GHS Classification:
- Skin irritation (Category 2)
- Eye irritation (Category 2A)
- Harmful to aquatic life
- Hazard Statements:
- H315: Causes skin irritation
- H319: Causes serious eye irritation
- H411: Toxic to aquatic life with long lasting effects
- PPE Requirements:
- Gloves (nitrile recommended)
- Protective goggles
- Lab coat
- Use in well-ventilated area or fume hood
- First Aid Measures:
- Inhalation: Move to fresh air
- Skin Contact: Wash with soap and water
- Eye Contact: Flush with water for several minutes
- Ingestion: Rinse mouth, seek medical attention
4. Storage & Handling Attributes
- Storage Conditions:
- Store in tightly closed containers
- Protect from light and heat
- Store components separately until ready for use
- Handling Notes:
- Avoid contact with eyes, skin, and clothing
- Mix just before use to ensure activity
5. Regulatory & Compliance Attributes
- Component CAS Numbers:
- Copper(II) sulfate pentahydrate: 7758-99-8
- Sodium potassium tartrate: 6381-59-5
- Sodium hydroxide: 1310-73-2
- EC Numbers:
- CuSO₄·5H₂O: 231-847-6
- Hazard Class (mixed): 8 (Corrosive)
- UN Numbers (if shipped mixed): UN 3264
6. Laboratory Applications
- Primary Uses:
- Qualitative test for reducing sugars (e.g., glucose, fructose, maltose)
- Used in school and biochemical labs for carbohydrate analysis
- Secondary Uses:
- Demonstrations in redox chemistry
- Educational reagent in classical qualitative analysis
SAFETY HANDLING PRECAUTIONS
SAFETY PRECAUTIONS
- Corrosive – due to NaOH in Solution 2
- Toxic to aquatic life – due to copper sulfate
- Use gloves, goggles, and lab coat
- Storage:
- Store separately in tightly closed bottles
- Keep in a cool, dry place away from light and incompatible substances
FIRST AID MEASURES
- Inhalation: Move to fresh air, seek medical attention if irritation persists
- Skin/Eye Contact: Rinse immediately with water for at least 15 minutes
- Ingestion: Rinse mouth, do not induce vomiting—seek urgent medical care
Related products
Aluminium Chloride Anhydrous Extra Pure
Aluminium Chloride Anhydrous Extra Pure is a high-purity, white to yellowish crystalline compound that is highly hygroscopic and fuming in moist air. It is extensively used in laboratory chemistry as a powerful Lewis acid catalyst, particularly in Friedel-Crafts alkylation and acylation reactions, as well as in the synthesis of organometallic compounds. Its anhydrous form ensures optimal reactivity and effectiveness in moisture-sensitive processes, including polymerization and halogenation studies. Due to its corrosive nature and tendency to react violently with water, it must be handled in a dry, controlled environment using proper safety measures. This extra pure grade is ideal for high-precision analytical and preparative procedures in organic and inorganic chemistry research.
Aluminium Ferric Sulphate Extra Pure
Aluminium Ferric Sulphate Extra Pure is a high-purity, yellowish to light brown crystalline solid composed of aluminum, iron, and sulfate ions, commonly used in laboratory settings for analytical, coordination, and inorganic chemistry research. Its dual-metal composition makes it valuable for studying metal ion interactions, flocculation processes, and as a reagent in qualitative analysis of phosphates and tannins. The compound also finds application in preparing standard solutions and in experiments related to coagulation and sedimentation. Its extra pure grade ensures consistent performance with minimal contamination, making it ideal for precise, controlled experimentation. It should be stored in tightly sealed containers in a dry, cool environment to maintain stability and prevent moisture uptake.
Ammonia Solution Extra Pure
Ammonia Solution Extra Pure is a high-purity, clear, colorless liquid composed of ammonia gas dissolved in water, emitting a strong, characteristic pungent odor. Widely used in laboratory chemistry, it serves as a crucial reagent in acid-base titrations, complexometric analysis, and the preparation of ammonium salts and metal-ammonia complexes. Its alkaline nature makes it valuable for pH adjustment, cleaning of laboratory glassware, and as a reducing agent in certain analytical procedures. The extra pure grade ensures low levels of impurities, supporting reliable results in sensitive experimental work. Due to its volatility and corrosiveness, it should be handled in well-ventilated areas and stored in tightly sealed, chemically resistant containers.
Ammonium Chromate Extra Pure
Ammonium Chromate Extra Pure is a high-purity, yellow crystalline compound primarily used in laboratory settings for analytical and inorganic chemistry applications. It serves as a strong oxidizing agent and is commonly utilized in qualitative analysis, particularly in the detection of cations, and in the preparation of chromate-based reagents. Its high solubility in water and ability to act as a corrosion inhibitor also make it useful in material science and electrochemical experiments. Due to the presence of hexavalent chromium, it is toxic and environmentally hazardous, requiring strict handling procedures and protective equipment. The extra pure grade ensures minimal impurities, making it suitable for high-precision research. It must be stored in tightly sealed containers, away from light, heat, and incompatible substances.
Ammonium Ferric Sulphate Extra Pure
Ammonium Ferric Sulphate Extra Pure, also known as ferric ammonium sulfate or iron alum, is a high-purity, violet to light purple crystalline compound used extensively in laboratory settings for analytical and inorganic chemistry. It serves as a reliable oxidizing agent and a standard in redox titrations, particularly in permanganometry. Its stable and non-hygroscopic nature makes it ideal for preparing standard iron solutions, studying coordination complexes, and teaching laboratory procedures involving iron(III) salts. The extra pure grade ensures minimal interference from impurities, enabling accurate and reproducible results in sensitive experiments. It should be stored in a dry, cool environment in well-sealed containers to preserve its chemical integrity.
Ammonium Ferrous Sulphate Extra Pure
Ammonium Ferrous Sulphate Extra Pure, also known as Mohr’s salt, is a high-purity, light green crystalline compound composed of ferrous sulfate and ammonium sulfate. It is widely used in laboratory settings as a stable source of ferrous ions for redox titrations, particularly in permanganometric and dichromate analyses. Its stability in air, compared to other iron(II) salts, makes it ideal for preparing standard solutions and studying oxidation-reduction reactions. This compound is also valuable in coordination chemistry and iron metabolism research. The extra pure grade ensures minimal contamination, supporting precise analytical and experimental results. It should be stored in tightly sealed containers in a cool, dry place to prevent oxidation and maintain its integrity.
Ammonium Iodide Extra Pure
Ammonium Iodide Extra Pure is a high-purity, white to slightly yellow crystalline compound highly soluble in water and alcohol, commonly used in laboratory chemistry for analytical, synthetic, and photographic applications. It serves as a valuable source of iodide ions in organic and inorganic reactions, including halide exchange and the preparation of iodine-containing compounds. In analytical chemistry, it is used in iodometric titrations and as a reagent for detecting metal ions. The extra pure grade ensures exceptional chemical stability and low levels of impurities, supporting accurate, reproducible results in sensitive experiments. It should be stored in tightly sealed containers, away from light and moisture, to prevent decomposition and discoloration.
Barium Sulphate Extra pure
Barium Sulphate Extra Pure is a high-purity, white, odorless crystalline powder known for its extreme insolubility in water and chemical inertness, making it highly useful in laboratory settings for qualitative analysis and gravimetric determinations. It is commonly employed in the detection and quantification of sulfate ions through precipitation reactions, as well as in studies involving solubility equilibria and surface chemistry. Its high density and stability also make it valuable in preparing calibration standards and radiopaque materials in research. The extra pure grade ensures minimal contamination, providing consistent and reliable results in sensitive analytical procedures. It should be stored in a dry, tightly sealed container to maintain its quality and prevent contamination.


 Preservatives(food)
Preservatives(food) Flavor Enhancers
Flavor Enhancers Acidulants
Acidulants Sweeteners
Sweeteners Antioxidants
Antioxidants Colorants(food)
Colorants(food) Nutraceutical Ingredients (food)
Nutraceutical Ingredients (food) Nutrient Supplements
Nutrient Supplements Emulsifiers
Emulsifiers
 Collectors
Collectors Dust Suppressants
Dust Suppressants Explosives and Blasting Agents
Explosives and Blasting Agents Flocculants and Coagulants
Flocculants and Coagulants Frothers
Frothers Leaching Agents
Leaching Agents pH Modifiers
pH Modifiers Precious Metal Extraction Agents
Precious Metal Extraction Agents
 Antioxidants(plastic)
Antioxidants(plastic) Colorants (Pigments, Dyes)
Colorants (Pigments, Dyes) Fillers and Reinforcements
Fillers and Reinforcements Flame Retardants
Flame Retardants Monomers
Monomers Plasticizers
Plasticizers Polymerization Initiators
Polymerization Initiators Stabilizers (UV, Heat)
Stabilizers (UV, Heat)
 Antifoaming Agents
Antifoaming Agents Chelating Agents
Chelating Agents Coagulants and Flocculants
Coagulants and Flocculants Corrosion Inhibitors
Corrosion Inhibitors Disinfectants and Biocides
Disinfectants and Biocides Oxidizing Agents
Oxidizing Agents pH Adjusters
pH Adjusters Scale Inhibitors( water)
Scale Inhibitors( water)
 Antioxidants(cosmetic)
Antioxidants(cosmetic) Emollients
Emollients Fragrances and Essential Oils
Fragrances and Essential Oils Humectants
Humectants Preservatives
Preservatives Surfactants(cosmetic)
Surfactants(cosmetic) Thickeners
Thickeners UV Filters
UV Filters
 Fertilizers
Fertilizers Soil Conditioners
Soil Conditioners Plant Growth Regulators
Plant Growth Regulators Animal Feed Additives
Animal Feed Additives Biostimulants
Biostimulants Pesticides (Herbicides, Insecticides, Fungicides)
Pesticides (Herbicides, Insecticides, Fungicides)
 Active Pharmaceutical Ingredients (APIs)
Active Pharmaceutical Ingredients (APIs) Excipients
Excipients Solvents(pharmaceutical)
Solvents(pharmaceutical) Antibiotics
Antibiotics Antiseptics and Disinfectants
Antiseptics and Disinfectants Vaccine Adjuvants
Vaccine Adjuvants Nutraceutical Ingredients (pharmaceutical)
Nutraceutical Ingredients (pharmaceutical) Analgesics & Antipyretics
Analgesics & Antipyretics
 Analytical Reagents
Analytical Reagents Solvents(lab)
Solvents(lab) Chromatography Chemicals
Chromatography Chemicals Spectroscopy Reagents
Spectroscopy Reagents microbiology-and-cell-culture-reagents
microbiology-and-cell-culture-reagents Molecular Biology Reagents
Molecular Biology Reagents Biochemical Reagents
Biochemical Reagents Inorganic and Organic Standards
Inorganic and Organic Standards Laboratory Safety Chemicals
Laboratory Safety Chemicals Specialty Laboratory Chemicals(Special Laboratory Equipment)
Specialty Laboratory Chemicals(Special Laboratory Equipment)
 Demulsifiers
Demulsifiers Hydraulic Fracturing Fluids
Hydraulic Fracturing Fluids Scale Inhibitors(oil)
Scale Inhibitors(oil) Surfactants(oil)
Surfactants(oil) Drilling Fluids
Drilling Fluids
 Dyes and Pigments
Dyes and Pigments Bleaching Agents
Bleaching Agents Softening Agents
Softening Agents Finishing Agents
Finishing Agents Antistatic Agents
Antistatic Agents
 Admixtures
Admixtures Waterproofing Agents
Waterproofing Agents Sealants and Adhesives
Sealants and Adhesives Curing Compounds
Curing Compounds Concrete Repair Chemicals
Concrete Repair Chemicals Anti-Corrosion Coatings
Anti-Corrosion Coatings
 Surfactants(cleaning)
Surfactants(cleaning) Builders
Builders Enzymes
Enzymes Solvents (Cleaning)
Solvents (Cleaning) Fragrances
Fragrances
 Electronic Chemicals
Electronic Chemicals Catalysts
Catalysts Lubricants
Lubricants Photographic Chemicals
Photographic Chemicals Refrigerants
Refrigerants Automotive chemicals
Automotive chemicals Pyrotechnic Chemicals
Pyrotechnic Chemicals
 Biodegradable Surfactants
Biodegradable Surfactants Bio-based Solvents
Bio-based Solvents Renewable Polymers
Renewable Polymers Carbon Capture Chemicals
Carbon Capture Chemicals Wastewater Treatment Chemicals
Wastewater Treatment Chemicals
 Pigments
Pigments Solvents(paint)
Solvents(paint) Specialty Coatings
Specialty Coatings Binders/Resins
Binders/Resins Additives
Additives Driers
Driers Anti-Corrosion Agents
Anti-Corrosion Agents Functional Coatings
Functional Coatings Application-Specific Coatings
Application-Specific Coatings
 Leavening Agents
Leavening Agents Dough Conditioners
Dough Conditioners Flour Treatments
Flour Treatments Fat Replacers
Fat Replacers Decoratives
Decoratives Preservatives(baking)
Preservatives(baking)
 Plasticizers & Softeners
Plasticizers & Softeners Reinforcing Agents
Reinforcing Agents Adhesion Promoters
Adhesion Promoters Vulcanizing Agents
Vulcanizing Agents Antidegradants
Antidegradants Blowing Agents
Blowing Agents Fillers & Extenders
Fillers & Extenders Accelerators & Retarders
Accelerators & Retarders