Back to products


Electrode Zinc Plate
$ 34.50 Original price was: $ 34.50.$ 34.43Current price is: $ 34.43.
Electrode Platinum Pair
$ 34.99 Original price was: $ 34.99.$ 34.83Current price is: $ 34.83.
Whatsapp Order
Electrode Platinum Pair consists of two high-purity platinum electrodes used in laboratory and industrial electrochemical applications. Renowned for their excellent chemical inertness, corrosion resistance, and superior electrical conductivity, platinum electrodes provide stable and reliable performance in harsh chemical environments. These electrodes are commonly employed in electrolysis, standard electrode potential measurements, and catalysis research. Typically designed as pairs, they facilitate accurate current flow and measurement in analytical and research settings, making them indispensable in pharmaceutical, environmental, and chemical laboratories.
Description
Table of Contents
Toggle
Electrode Platinum Pair
Primary Uses
- Electrochemical and Analytical Applications
- Used as inert electrodes in electrolysis for decomposition of chemical compounds.
- Employed in potentiometric measurements and as reference electrodes in pH meters and other electrochemical sensors.
- Facilitates catalytic reactions in research involving oxidation-reduction processes.
- Laboratory and Research Uses
- Standard electrodes for calibration in analytical chemistry experiments.
- Utilized in voltammetry and other electrochemical analysis techniques.
Secondary Uses
- Industrial and Environmental Applications
- Applied in electroplating processes requiring high corrosion resistance electrodes.
- Used in environmental monitoring instruments for detecting chemical species.
- Educational Use
- Demonstrates electrochemical principles in academic laboratories.
KEY PRODUCT FEATURES
1.Basic Identification Attributes
- Material: High-purity platinum metal.
- Configuration: Paired electrodes designed for insertion into electrochemical cells.
- Sizes: Available in various dimensions depending on application requirements.
2.Physical & Chemical Properties
- Chemical Resistance: Outstanding resistance to oxidation, acids, and corrosive media.
- Electrical Conductivity: Excellent, providing precise measurement and consistent current flow.
- Thermal Stability: Can withstand high temperatures without degradation.
3.Safety & Hazard Attributes
- Non-toxic and chemically inert; safe for handling under normal laboratory conditions.
- Handle with care to avoid physical damage or contamination.
4.Storage & Handling Attributes
- Store in clean, dry environments to maintain electrode integrity.
- Avoid contact with abrasive materials that can scratch or damage the platinum surface.
- Use protective covers or cases when not in use.
5.Regulatory & Compliance Attributes
- Manufactured to meet laboratory standards for electrochemical instrumentation.
- Compliant with environmental and safety regulations for chemical laboratory equipment.
6.Environmental & Health Impact
- Platinum is recyclable and environmentally sustainable.
- Proper disposal or recycling of platinum electrodes helps conserve precious metals.
SAFETY HANDLING PRECAUTIONS
Safety Handling Precautions
- Use gloves to prevent contamination of the electrode surface.
- Handle carefully to avoid bending or damaging the electrodes.
First Aid Measures
- No specific hazards under normal handling; treat any physical injuries from sharp edges normally.
Firefighting Measures
- Non-flammable metal; use appropriate extinguishing methods for surrounding materials in case of fire.
Related products
Absorption Tower
An Absorption Tower is a vertical vessel used in industrial processes to remove specific components from gas streams by contact with a liquid solvent. This equipment facilitates mass transfer between the gas and liquid phases, allowing targeted pollutants or valuable compounds to be absorbed efficiently. Absorption Towers are commonly employed in chemical plants, refineries, and environmental control systems for gas scrubbing, purification, and recovery applications. They are designed for optimal gas-liquid contact, often using packing materials or trays to enhance surface area and improve absorption efficiency.
Bare Enamelled Copper Wire
Bare Enamelled Copper Wire is a high-quality electrical conductor coated with a thin layer of insulating enamel. It is widely used in electrical and electronic applications where insulation and durability are required without adding bulk. The enamel coating provides excellent resistance to heat, abrasion, and chemicals, making it suitable for winding coils, transformers, motors, and inductors. The bare enamelled copper wire ensures efficient conductivity combined with insulation properties, ideal for both laboratory research and industrial manufacturing processes.
Bernoulli Tube Apparatus
Bernoulli Tube Apparatus is a precision-engineered device designed to demonstrate and analyze the principles of fluid dynamics, specifically Bernoulli’s theorem. It consists of a transparent, variable-diameter horizontal tube equipped with multiple pressure tap points connected to manometer tubes. This allows for the visual observation and quantitative measurement of pressure, velocity, and flow relationships as fluid moves through different cross-sectional areas. Commonly used in laboratory and industrial fluid mechanics testing, it helps validate theoretical predictions in controlled experimental conditions.
Bio-Waste Digester Kit
The Bio-Waste Digester Kit (1 Liter) is a compact, ready-to-use microbial solution designed to accelerate the decomposition of organic waste through natural biodegradation. This kit contains a high-activity blend of non-pathogenic microorganisms and enzymes capable of breaking down a wide range of biodegradable materials including food scraps, plant matter, and organic sludge. It is formulated for efficient use in laboratory-scale digestion experiments as well as small-scale industrial or institutional bio-waste treatment systems. The product supports environmentally responsible waste management by reducing organic load, minimizing odors, and converting waste into more manageable byproducts such as methane, carbon dioxide, and stabilized compost.
Crookes radiometer
The Crookes Radiometer, also known as a light mill, is a scientific apparatus consisting of a glass bulb containing a partial vacuum and a rotor with vanes coated black on one side and white or silver on the other. When exposed to light or radiant energy, the vanes rotate due to differential thermal transpiration, demonstrating principles of gas kinetics and energy conversion. It is commonly used for educational demonstrations of light pressure and thermodynamics.
Crucible Tongs
Crucible Tongs are specialized metal tools designed for safely handling hot crucibles, evaporating dishes, and other laboratory apparatus exposed to high temperatures. Made from durable, heat-resistant metals such as stainless steel or nickel-plated steel, these tongs provide a firm grip, ensuring the safe transfer of heated items during laboratory procedures, including heating, melting, and chemical reactions.
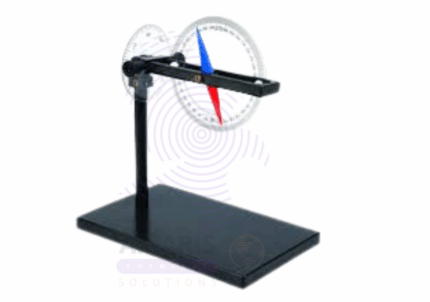
Dip Circle
A Dip Circle is a precision scientific instrument used for measuring the magnetic dip or inclination angle of the Earth’s magnetic field at a specific location. It consists of a magnetic needle or dip needle mounted on a graduated circular scale, which can rotate freely in the vertical plane. The instrument allows geologists, physicists, and surveyors to determine the angle between the horizontal plane and the Earth’s magnetic field lines, an important parameter in geomagnetic studies and navigation. Dip circles are commonly employed in academic research, mineral exploration, and educational demonstrations to analyze variations in Earth’s magnetism and assist in directional orientation.
Dip Needle
A Dip Needle is a finely balanced magnetic needle used to measure the magnetic dip or inclination angle of the Earth’s magnetic field. Mounted to pivot freely in a vertical plane, the needle aligns itself with the Earth’s magnetic field lines, enabling precise determination of the angle between the horizontal plane and magnetic field direction. Dip Needles are essential instruments in geophysics, navigation, and laboratory studies of magnetism. They provide crucial data for mapping magnetic variations, compass calibration, and educational demonstrations. Typically constructed with magnetized steel or alloy, dip needles are designed for sensitivity and durability in field and lab environments.


 Preservatives(food)
Preservatives(food) Flavor Enhancers
Flavor Enhancers Acidulants
Acidulants Sweeteners
Sweeteners Antioxidants
Antioxidants Colorants(food)
Colorants(food) Nutraceutical Ingredients (food)
Nutraceutical Ingredients (food) Nutrient Supplements
Nutrient Supplements Emulsifiers
Emulsifiers
 Collectors
Collectors Dust Suppressants
Dust Suppressants Explosives and Blasting Agents
Explosives and Blasting Agents Flocculants and Coagulants
Flocculants and Coagulants Frothers
Frothers Leaching Agents
Leaching Agents pH Modifiers
pH Modifiers Precious Metal Extraction Agents
Precious Metal Extraction Agents
 Antioxidants(plastic)
Antioxidants(plastic) Colorants (Pigments, Dyes)
Colorants (Pigments, Dyes) Fillers and Reinforcements
Fillers and Reinforcements Flame Retardants
Flame Retardants Monomers
Monomers Plasticizers
Plasticizers Polymerization Initiators
Polymerization Initiators Stabilizers (UV, Heat)
Stabilizers (UV, Heat)
 Antifoaming Agents
Antifoaming Agents Chelating Agents
Chelating Agents Coagulants and Flocculants
Coagulants and Flocculants Corrosion Inhibitors
Corrosion Inhibitors Disinfectants and Biocides
Disinfectants and Biocides Oxidizing Agents
Oxidizing Agents pH Adjusters
pH Adjusters Scale Inhibitors( water)
Scale Inhibitors( water)
 Antioxidants(cosmetic)
Antioxidants(cosmetic) Emollients
Emollients Fragrances and Essential Oils
Fragrances and Essential Oils Humectants
Humectants Preservatives
Preservatives Surfactants(cosmetic)
Surfactants(cosmetic) Thickeners
Thickeners UV Filters
UV Filters
 Fertilizers
Fertilizers Soil Conditioners
Soil Conditioners Plant Growth Regulators
Plant Growth Regulators Animal Feed Additives
Animal Feed Additives Biostimulants
Biostimulants Pesticides (Herbicides, Insecticides, Fungicides)
Pesticides (Herbicides, Insecticides, Fungicides)
 Active Pharmaceutical Ingredients (APIs)
Active Pharmaceutical Ingredients (APIs) Excipients
Excipients Solvents(pharmaceutical)
Solvents(pharmaceutical) Antibiotics
Antibiotics Antiseptics and Disinfectants
Antiseptics and Disinfectants Vaccine Adjuvants
Vaccine Adjuvants Nutraceutical Ingredients (pharmaceutical)
Nutraceutical Ingredients (pharmaceutical) Analgesics & Antipyretics
Analgesics & Antipyretics
 Analytical Reagents
Analytical Reagents Solvents(lab)
Solvents(lab) Chromatography Chemicals
Chromatography Chemicals Spectroscopy Reagents
Spectroscopy Reagents microbiology-and-cell-culture-reagents
microbiology-and-cell-culture-reagents Molecular Biology Reagents
Molecular Biology Reagents Biochemical Reagents
Biochemical Reagents Inorganic and Organic Standards
Inorganic and Organic Standards Laboratory Safety Chemicals
Laboratory Safety Chemicals Specialty Laboratory Chemicals(Special Laboratory Equipment)
Specialty Laboratory Chemicals(Special Laboratory Equipment)
 Demulsifiers
Demulsifiers Hydraulic Fracturing Fluids
Hydraulic Fracturing Fluids Scale Inhibitors(oil)
Scale Inhibitors(oil) Surfactants(oil)
Surfactants(oil) Drilling Fluids
Drilling Fluids
 Dyes and Pigments
Dyes and Pigments Bleaching Agents
Bleaching Agents Softening Agents
Softening Agents Finishing Agents
Finishing Agents Antistatic Agents
Antistatic Agents
 Admixtures
Admixtures Waterproofing Agents
Waterproofing Agents Sealants and Adhesives
Sealants and Adhesives Curing Compounds
Curing Compounds Concrete Repair Chemicals
Concrete Repair Chemicals Anti-Corrosion Coatings
Anti-Corrosion Coatings
 Surfactants(cleaning)
Surfactants(cleaning) Builders
Builders Enzymes
Enzymes Solvents (Cleaning)
Solvents (Cleaning) Fragrances
Fragrances
 Electronic Chemicals
Electronic Chemicals Catalysts
Catalysts Lubricants
Lubricants Photographic Chemicals
Photographic Chemicals Refrigerants
Refrigerants Automotive chemicals
Automotive chemicals Pyrotechnic Chemicals
Pyrotechnic Chemicals
 Biodegradable Surfactants
Biodegradable Surfactants Bio-based Solvents
Bio-based Solvents Renewable Polymers
Renewable Polymers Carbon Capture Chemicals
Carbon Capture Chemicals Wastewater Treatment Chemicals
Wastewater Treatment Chemicals
 Pigments
Pigments Solvents(paint)
Solvents(paint) Specialty Coatings
Specialty Coatings Binders/Resins
Binders/Resins Additives
Additives Driers
Driers Anti-Corrosion Agents
Anti-Corrosion Agents Functional Coatings
Functional Coatings Application-Specific Coatings
Application-Specific Coatings
 Fresh Herbs
Fresh Herbs Ground Spices
Ground Spices Whole Spices
Whole Spices Spice Blends
Spice Blends Dried Herbs
Dried Herbs
 Leavening Agents
Leavening Agents Dough Conditioners
Dough Conditioners Flour Treatments
Flour Treatments Fat Replacers
Fat Replacers Decoratives
Decoratives Preservatives(baking)
Preservatives(baking)
 Plasticizers & Softeners
Plasticizers & Softeners Reinforcing Agents
Reinforcing Agents Adhesion Promoters
Adhesion Promoters Vulcanizing Agents
Vulcanizing Agents Antidegradants
Antidegradants Blowing Agents
Blowing Agents Fillers & Extenders
Fillers & Extenders Accelerators & Retarders
Accelerators & Retarders