“Albumen From Egg Powder Extra Pure” has been added to your cart. View cart
Back to products


Ferric Chloride Anhydrous Extra Pure
$ 18.00 Original price was: $ 18.00.$ 17.90Current price is: $ 17.90.
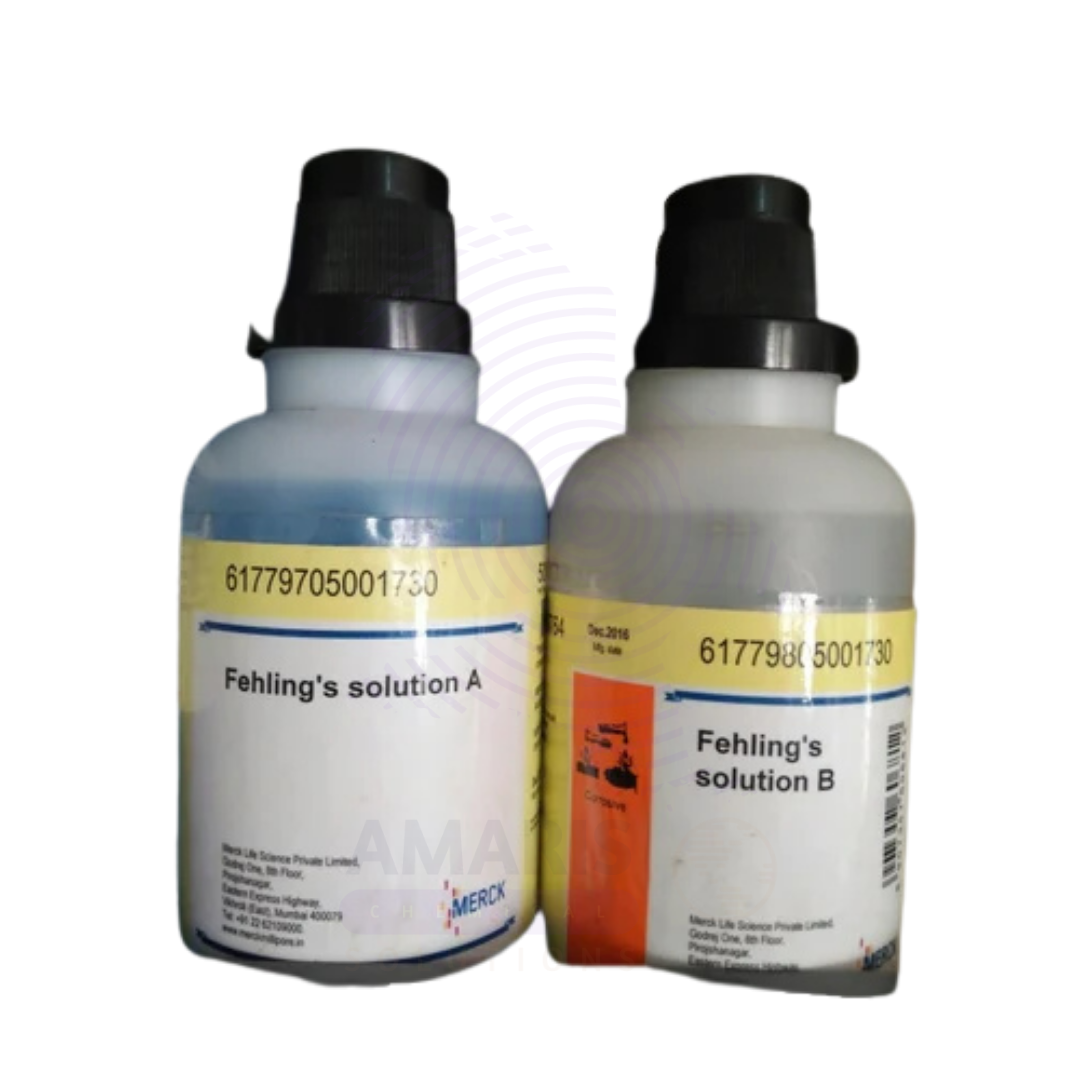
Fehling Solution 1 and 2 Extra Pure
$ 36.00 Original price was: $ 36.00.$ 35.90Current price is: $ 35.90.
Whatsapp Order
Fehling Solution 1 and 2 Extra Pure are essential reagents in classical laboratory chemistry, particularly used for the qualitative detection of reducing sugars such as glucose.
- Fehling Solution 1 contains copper(II) sulfate, while
- Fehling Solution 2 contains an alkaline potassium sodium tartrate (Rochelle salt) solution.
When mixed in equal parts just before use, they form a deep blue complex that, upon heating with a reducing sugar, yields a brick-red precipitate of cuprous oxide, confirming the presence of aldehyde groups.
These solutions are highly valuable in educational labs, food testing, and biochemical experiments. Store them separately in cool, dry conditions, and always mix fresh for accurate results.
Description
Table of Contents
Toggle
Fehling Solution 1 and 2 Extra Pure
Primary Uses
- Detection of reducing sugars:
Widely used in qualitative analysis to test for the presence of aldehyde functional groups, especially in reducing sugars like glucose. - Differentiation between aldehydes and ketones:
Reacts with aldehydes to form a red precipitate of copper(I) oxide, while ketones generally do not react. - Educational experiments in redox chemistry:
Demonstrates redox reactions and the principle of oxidation-reduction using visible color change.
Secondary Uses
- Identification of carbohydrate content in unknown samples:
Used to confirm the presence of reducing sugars in plant extracts, juices, or hydrolyzed polysaccharides. - Routine qualitative analysis in food chemistry labs:
Applied in basic food testing kits and protocols to check sugar levels in various food products. - Historical method reference in pharmaceutical analysis:
Though mostly replaced by modern techniques, still referenced in classic pharmaceutical and biochemical protocols.
KEY PRODUCT FEATURES
KEY ATTRIBUTES
1. Basic Identification Attributes
- Chemical Name: Fehling Solution (Part I & II)
- Components:
- Fehling’s Solution I: Aqueous copper(II) sulfate (CuSO₄·5H₂O) solution
- Fehling’s Solution II: Alkaline sodium potassium tartrate (Rochelle salt) in sodium hydroxide solution
- Grade: Extra Pure (Laboratory Reagent Grade)
- Appearance:
- Solution I: Clear blue solution
- Solution II: Colorless or pale solution
2. Physical & Chemical Properties
- Fehling’s Solution I:
- Copper(II) sulfate concentration: Typically ~0.5 M
- Fehling’s Solution II:
- Sodium potassium tartrate: Chelates the copper ions
- Sodium hydroxide concentration: ~1.5 M
- When mixed (equal volumes of I & II): Deep blue complex due to formation of a copper-tartrate complex
3. Safety & Hazard Attributes
- GHS Classification:
- Skin irritation (Category 2)
- Eye irritation (Category 2A)
- Harmful to aquatic life
- Hazard Statements:
- H315: Causes skin irritation
- H319: Causes serious eye irritation
- H411: Toxic to aquatic life with long lasting effects
- PPE Requirements:
- Gloves (nitrile recommended)
- Protective goggles
- Lab coat
- Use in well-ventilated area or fume hood
- First Aid Measures:
- Inhalation: Move to fresh air
- Skin Contact: Wash with soap and water
- Eye Contact: Flush with water for several minutes
- Ingestion: Rinse mouth, seek medical attention
4. Storage & Handling Attributes
- Storage Conditions:
- Store in tightly closed containers
- Protect from light and heat
- Store components separately until ready for use
- Handling Notes:
- Avoid contact with eyes, skin, and clothing
- Mix just before use to ensure activity
5. Regulatory & Compliance Attributes
- Component CAS Numbers:
- Copper(II) sulfate pentahydrate: 7758-99-8
- Sodium potassium tartrate: 6381-59-5
- Sodium hydroxide: 1310-73-2
- EC Numbers:
- CuSO₄·5H₂O: 231-847-6
- Hazard Class (mixed): 8 (Corrosive)
- UN Numbers (if shipped mixed): UN 3264
6. Laboratory Applications
- Primary Uses:
- Qualitative test for reducing sugars (e.g., glucose, fructose, maltose)
- Used in school and biochemical labs for carbohydrate analysis
- Secondary Uses:
- Demonstrations in redox chemistry
- Educational reagent in classical qualitative analysis
SAFETY HANDLING PRECAUTIONS
SAFETY MEASURES
SAFETY & HANDLING
- Corrosive – due to NaOH in Solution 2
- Toxic to aquatic life – due to copper sulfate
- Use gloves, goggles, and lab coat
- Storage:
- Store separately in tightly closed bottles
- Keep in a cool, dry place away from light and incompatible substances
FIRST AID MEASURES
- Inhalation: Move to fresh air, seek medical attention if irritation persists
- Skin/Eye Contact: Rinse immediately with water for at least 15 minutes
- Ingestion: Rinse mouth, do not induce vomiting—seek urgent medical care
Related products
Alizarin Extra Pure
Alizarin Extra Pure is a high-grade anthraquinone-based dye, presented as a reddish-brown crystalline powder, renowned for its vivid coloration and strong affinity for metal ions. It is primarily used in analytical chemistry as a complexometric indicator, especially for detecting aluminum, calcium, and other metal ions through color change. Due to its exceptional purity, it is also employed in histological staining, textile research, and pigment synthesis where precision and consistency are critical. Alizarin Extra Pure offers excellent stability and intense coloration, making it ideal for scientific, educational, and specialized industrial applications. Proper storage in a cool, dry environment is essential to maintain its effectiveness and prevent degradation.
Aluminium Ferric Sulphate Extra Pure
Aluminium Ferric Sulphate Extra Pure is a high-purity, yellowish to light brown crystalline solid composed of aluminum, iron, and sulfate ions, commonly used in laboratory settings for analytical, coordination, and inorganic chemistry research. Its dual-metal composition makes it valuable for studying metal ion interactions, flocculation processes, and as a reagent in qualitative analysis of phosphates and tannins. The compound also finds application in preparing standard solutions and in experiments related to coagulation and sedimentation. Its extra pure grade ensures consistent performance with minimal contamination, making it ideal for precise, controlled experimentation. It should be stored in tightly sealed containers in a dry, cool environment to maintain stability and prevent moisture uptake.
Aluminium Metal Fine Extra Pure
Aluminium Metal Fine Extra Pure is a high-purity, silvery-grey powder composed of finely divided aluminum particles, ideal for precision laboratory work and specialized chemical reactions. Known for its high surface area and excellent reactivity, it is widely used in thermite reactions, metallurgical experiments, and the synthesis of aluminum-based compounds. Its fine particle size enhances its role as a reducing agent in organic and inorganic chemistry, as well as in combustion and pyrotechnic research. The extra pure grade ensures minimal trace impurities, supporting consistent and accurate results in sensitive analytical and preparative procedures. Due to its flammability and potential for dust explosions, it must be handled with caution in well-ventilated areas and stored in sealed, moisture-free containers.
Aluminium Oxide Active Neutral Extra Pure
Aluminium Oxide Active Neutral Extra Pure is a high-purity, fine white powder known for its high surface area and neutral pH, making it ideal for chromatography, adsorption, and catalyst support in laboratory research. Its active form ensures excellent capacity for separating and purifying organic compounds without altering their chemical structure, especially in column chromatography applications. Commonly used in analytical, organic synthesis, and material science laboratories, it provides consistent performance in adsorption studies, drying of solvents, and reaction monitoring. The extra pure grade ensures extremely low levels of impurities, suitable for high-precision experimental work. To maintain its activity, it should be stored in airtight containers away from moisture and reactive chemicals.
Ammonium Chloride Extra Pure
Ammonium Chloride Extra Pure is a high-purity, white crystalline salt widely used in laboratory chemistry as a source of ammonium ions and chloride ions for analytical, inorganic, and biochemical applications. It plays a key role in preparing buffer solutions, especially in conjunction with ammonia, and is commonly used in qualitative analysis, electrochemical studies, and metal treatment experiments. In addition, it serves as a reagent in synthesis reactions and as a nitrogen source in microbial culture media. The extra pure grade ensures high consistency and minimal contaminants, making it suitable for precise and sensitive laboratory work. It should be stored in a cool, dry place in well-sealed containers to maintain stability and prevent moisture absorption.
Ammonium Cupric Chloride Extra Pure
- SHORT DESCRIPTION
N propyl Acetate
N propyl Acetate is a clear, colorless, flammable liquid ester with a fruity odor, chemically known as propyl ethanoate. It is widely used as a solvent in coatings, inks, adhesives, and cleaning agents due to its excellent solvency and moderate evaporation rate. N-Propyl Acetate is valued for its ability to dissolve various resins and polymers, providing good film formation and gloss in paints and coatings.
Phosphoric Acid Food Grade
Phosphoric Acid Food Grade is a highly concentrated, colorless, odorless liquid acid used extensively in the food and beverage industry. It acts as an acidulant, flavoring agent, and preservative, approved for direct use in food processing. This grade meets strict purity standards suitable for consumption and is widely employed to control pH, add tanginess, and extend shelf life. It is also used in other industries requiring high-purity phosphoric acid.


 Acidulants
Acidulants Antioxidants
Antioxidants Nutraceutical Ingredients (food)
Nutraceutical Ingredients (food)
 Collectors
Collectors Dust Suppressants
Dust Suppressants Explosives and Blasting Agents
Explosives and Blasting Agents Flocculants and Coagulants
Flocculants and Coagulants Frothers
Frothers Leaching Agents
Leaching Agents pH Modifiers
pH Modifiers Precious Metal Extraction Agents
Precious Metal Extraction Agents
 Antioxidants(plastic)
Antioxidants(plastic) Colorants (Pigments, Dyes)
Colorants (Pigments, Dyes) Fillers and Reinforcements
Fillers and Reinforcements Flame Retardants
Flame Retardants Monomers
Monomers Plasticizers
Plasticizers Polymerization Initiators
Polymerization Initiators Stabilizers (UV, Heat)
Stabilizers (UV, Heat)
 Antifoaming Agents
Antifoaming Agents Chelating Agents
Chelating Agents Coagulants and Flocculants
Coagulants and Flocculants Corrosion Inhibitors
Corrosion Inhibitors Disinfectants and Biocides
Disinfectants and Biocides Oxidizing Agents
Oxidizing Agents pH Adjusters
pH Adjusters Scale Inhibitors( water)
Scale Inhibitors( water)
 Antioxidants(cosmetic)
Antioxidants(cosmetic) Emollients
Emollients Fragrances and Essential Oils
Fragrances and Essential Oils Humectants
Humectants Preservatives
Preservatives Surfactants(cosmetic)
Surfactants(cosmetic) Thickeners
Thickeners UV Filters
UV Filters
 Fertilizers
Fertilizers Soil Conditioners
Soil Conditioners Plant Growth Regulators
Plant Growth Regulators Animal Feed Additives
Animal Feed Additives Biostimulants
Biostimulants Pesticides (Herbicides, Insecticides, Fungicides)
Pesticides (Herbicides, Insecticides, Fungicides)
 Active Pharmaceutical Ingredients (APIs)
Active Pharmaceutical Ingredients (APIs) Excipients
Excipients Solvents(pharmaceutical)
Solvents(pharmaceutical) Antibiotics
Antibiotics Antiseptics and Disinfectants
Antiseptics and Disinfectants Vaccine Adjuvants
Vaccine Adjuvants Nutraceutical Ingredients (pharmaceutical)
Nutraceutical Ingredients (pharmaceutical) Analgesics & Antipyretics
Analgesics & Antipyretics
 Analytical Reagents
Analytical Reagents Chromatography Chemicals
Chromatography Chemicals Spectroscopy Reagents
Spectroscopy Reagents Molecular Biology Reagents
Molecular Biology Reagents Biochemical Reagents
Biochemical Reagents Inorganic and Organic Standards
Inorganic and Organic Standards Laboratory Safety Chemicals
Laboratory Safety Chemicals Specialty Laboratory Chemicals(Special Laboratory Equipment)
Specialty Laboratory Chemicals(Special Laboratory Equipment)
 Demulsifiers
Demulsifiers Hydraulic Fracturing Fluids
Hydraulic Fracturing Fluids Scale Inhibitors(oil)
Scale Inhibitors(oil) Surfactants(oil)
Surfactants(oil) Drilling Fluids
Drilling Fluids
 Dyes and Pigments
Dyes and Pigments Bleaching Agents
Bleaching Agents Softening Agents
Softening Agents Finishing Agents
Finishing Agents Antistatic Agents
Antistatic Agents
 Admixtures
Admixtures Waterproofing Agents
Waterproofing Agents Sealants and Adhesives
Sealants and Adhesives Curing Compounds
Curing Compounds Concrete Repair Chemicals
Concrete Repair Chemicals Anti-Corrosion Coatings
Anti-Corrosion Coatings
 Surfactants(cleaning)
Surfactants(cleaning) Builders
Builders Enzymes
Enzymes Solvents (Cleaning)
Solvents (Cleaning) Fragrances
Fragrances
 Electronic Chemicals
Electronic Chemicals Catalysts
Catalysts Lubricants
Lubricants Photographic Chemicals
Photographic Chemicals Refrigerants
Refrigerants Automotive chemicals
Automotive chemicals Pyrotechnic Chemicals
Pyrotechnic Chemicals
 Biodegradable Surfactants
Biodegradable Surfactants Bio-based Solvents
Bio-based Solvents Renewable Polymers
Renewable Polymers Carbon Capture Chemicals
Carbon Capture Chemicals Wastewater Treatment Chemicals
Wastewater Treatment Chemicals
 Pigments
Pigments Solvents(paint)
Solvents(paint) Specialty Coatings
Specialty Coatings Binders/Resins
Binders/Resins Additives
Additives Driers
Driers Anti-Corrosion Agents
Anti-Corrosion Agents Functional Coatings
Functional Coatings Application-Specific Coatings
Application-Specific Coatings
 Fresh Herbs
Fresh Herbs Ground Spices
Ground Spices Whole Spices
Whole Spices Spice Blends
Spice Blends Dried Herbs
Dried Herbs
 Leavening Agents
Leavening Agents Dough Conditioners
Dough Conditioners Flour Treatments
Flour Treatments Fat Replacers
Fat Replacers Decoratives
Decoratives Preservatives(baking)
Preservatives(baking)
 Plasticizers & Softeners
Plasticizers & Softeners Reinforcing Agents
Reinforcing Agents Adhesion Promoters
Adhesion Promoters Vulcanizing Agents
Vulcanizing Agents Antidegradants
Antidegradants Blowing Agents
Blowing Agents Fillers & Extenders
Fillers & Extenders Accelerators & Retarders
Accelerators & Retarders