
 AGRICULTURAL CHEMICALS37 products
AGRICULTURAL CHEMICALS37 products
 BAKING INGREDIENTS8 products
BAKING INGREDIENTS8 products
 CLEANING AND DETERGENT CHEMICALS95 products
CLEANING AND DETERGENT CHEMICALS95 products
 CONSTRUCTION CHEMICALS43 products
CONSTRUCTION CHEMICALS43 products
 COSMETIC AND PERSONAL CARE CHEMICALS336 products
COSMETIC AND PERSONAL CARE CHEMICALS336 products
 ENVIRONMENTAL AND GREEN CHEMICALS2 products
ENVIRONMENTAL AND GREEN CHEMICALS2 products
 FOOD AND BEVERAGE CHEMICALS293 products
FOOD AND BEVERAGE CHEMICALS293 products
 HERBS & SPICES7 products
HERBS & SPICES7 products
 LABORATORY CHEMICALS363 products
LABORATORY CHEMICALS363 products
 Analytical Reagents281 products
Analytical Reagents281 products
 Biochemical Reagents114 products
Biochemical Reagents114 products
 Chromatography Chemicals6 products
Chromatography Chemicals6 products
 Inorganic and Organic Standards98 products
Inorganic and Organic Standards98 products
 Laboratory Safety Chemicals52 products
Laboratory Safety Chemicals52 products
 microbiology-and-cell-culture-reagents28 products
microbiology-and-cell-culture-reagents28 products
 Molecular Biology Reagents5 products
Molecular Biology Reagents5 products
 Solvents(lab)37 products
Solvents(lab)37 products
 Specialty Laboratory Chemicals(Special Laboratory Equipment)46 products
Specialty Laboratory Chemicals(Special Laboratory Equipment)46 products
 Spectroscopy Reagents5 products
Spectroscopy Reagents5 products
 LABORATORY EQUIPMENT & APPARATUS272 products
LABORATORY EQUIPMENT & APPARATUS272 products
 General Lab Glassware & Plasticware78 products
General Lab Glassware & Plasticware78 products
 Heating & Cooling Equipment5 products
Heating & Cooling Equipment5 products
 Lab Furniture & Storage Solutions14 products
Lab Furniture & Storage Solutions14 products
 Lab Safety & Protective Gear6 products
Lab Safety & Protective Gear6 products
 Measuring & Analytical Instruments57 products
Measuring & Analytical Instruments57 products
 microscopy-imaging-systems7 products
microscopy-imaging-systems7 products
 Mixing & Separation Devices15 products
Mixing & Separation Devices15 products
 Specialized Scientific Instruments89 products
Specialized Scientific Instruments89 products
 MINING CHEMICALS22 products
MINING CHEMICALS22 products
 OIL AND GAS CHEMICALS3 products
OIL AND GAS CHEMICALS3 products
 OTHERS3 products
OTHERS3 products
 PAINT & COATINGS CHEMICALS113 products
PAINT & COATINGS CHEMICALS113 products
 PHARMACEUTICAL CHEMICALS167 products
PHARMACEUTICAL CHEMICALS167 products
 Active Pharmaceutical Ingredients (APIs)97 products
Active Pharmaceutical Ingredients (APIs)97 products
 Analgesics & Antipyretics0 products
Analgesics & Antipyretics0 products
 Antibiotics2 products
Antibiotics2 products
 Antiseptics and Disinfectants16 products
Antiseptics and Disinfectants16 products
 Excipients33 products
Excipients33 products
 Nutraceutical Ingredients (pharmaceutical)16 products
Nutraceutical Ingredients (pharmaceutical)16 products
 Solvents(pharmaceutical)5 products
Solvents(pharmaceutical)5 products
 Topical Steroids1 product
Topical Steroids1 product
 Vaccine Adjuvants0 products
Vaccine Adjuvants0 products
 PLASTICS AND POLYMER MANUFACTURING CHEMICALS82 products
PLASTICS AND POLYMER MANUFACTURING CHEMICALS82 products
 RUBBER PROCESSING CHEMICALS23 products
RUBBER PROCESSING CHEMICALS23 products
 SPECIALTY AND FINE CHEMICALS46 products
SPECIALTY AND FINE CHEMICALS46 products
 TEXTILE CHEMICALS55 products
TEXTILE CHEMICALS55 products
 WATER TREATMENT CHEMICALS70 products
WATER TREATMENT CHEMICALS70 products
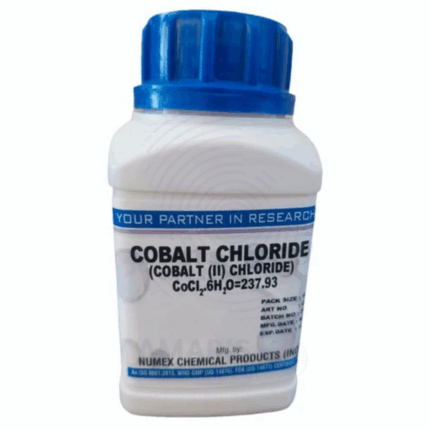
Cobalt Chloride Extra Pure
Cobalt Chloride Extra Pure is a high-purity, pink to deep purple crystalline compound widely used in laboratories for analytical, inorganic, and coordination chemistry. It is especially valued as a humidity indicator, turning from blue (anhydrous) to pink (hydrated) depending on moisture levels. In research settings, it serves as a precursor for synthesizing other cobalt compounds, as well as a reagent in qualitative tests for water, ammonium ions, and complex formation studies. Its extra pure grade ensures precise, contamination-free results, making it ideal for experiments requiring high accuracy. Proper handling with gloves and storage in airtight containers are recommended due to its toxic and moisture-sensitive nature.
Copper Hydroxide Extra Pure
Copper Hydroxide Extra Pure is a finely powdered, blue-green compound used in laboratory settings for analytical, inorganic, and qualitative chemistry applications. Its high purity makes it ideal for precipitation reactions, synthesis of copper-based compounds, and studies involving hydroxide ion behavior. In educational labs, it demonstrates typical base-metal hydroxide characteristics, such as low solubility and reactivity with acids to form copper salts. Due to its instability under light and air, it should be handled with care and stored in well-sealed, light-resistant containers to maintain its integrity for reliable experimental outcomes.
Nickel Sulphate Extra Pure
Nickel Sulphate Extra Pure is a crystalline, green-colored compound of high purity, widely utilized in analytical, electrochemical, and industrial applications. It is primarily used in electroplating processes to deposit a uniform layer of nickel onto metal surfaces, enhancing corrosion resistance and aesthetic appeal. Its consistent composition makes it ideal for high-precision electroforming and battery component manufacturing, especially in nickel-cadmium and nickel-metal hydride batteries.
In laboratories, it serves as a reagent for chemical analysis, particularly in qualitative and quantitative detection of nickel ions. It is also involved in catalyst production, ceramic coloring, and metal surface treatment. The Extra Pure grade guarantees minimal impurities, ensuring reliable performance in sensitive experimental setups. Due to its toxic and irritant nature, it should be handled with appropriate personal protective equipment and stored in a cool, dry place in tightly closed containers.

 Preservatives(food)
Preservatives(food) Flavor Enhancers
Flavor Enhancers Acidulants
Acidulants Sweeteners
Sweeteners Antioxidants
Antioxidants Colorants(food)
Colorants(food) Nutraceutical Ingredients (food)
Nutraceutical Ingredients (food) Nutrient Supplements
Nutrient Supplements Emulsifiers
Emulsifiers Collectors
Collectors Dust Suppressants
Dust Suppressants Explosives and Blasting Agents
Explosives and Blasting Agents Flocculants and Coagulants
Flocculants and Coagulants Frothers
Frothers Leaching Agents
Leaching Agents pH Modifiers
pH Modifiers Precious Metal Extraction Agents
Precious Metal Extraction Agents Antioxidants(plastic)
Antioxidants(plastic) Colorants (Pigments, Dyes)
Colorants (Pigments, Dyes) Fillers and Reinforcements
Fillers and Reinforcements Flame Retardants
Flame Retardants Monomers
Monomers Plasticizers
Plasticizers Polymerization Initiators
Polymerization Initiators Stabilizers (UV, Heat)
Stabilizers (UV, Heat) Antifoaming Agents
Antifoaming Agents Chelating Agents
Chelating Agents Coagulants and Flocculants
Coagulants and Flocculants Corrosion Inhibitors
Corrosion Inhibitors Disinfectants and Biocides
Disinfectants and Biocides Oxidizing Agents
Oxidizing Agents pH Adjusters
pH Adjusters Scale Inhibitors( water)
Scale Inhibitors( water) Antioxidants(cosmetic)
Antioxidants(cosmetic) Emollients
Emollients Fragrances and Essential Oils
Fragrances and Essential Oils Humectants
Humectants Preservatives
Preservatives Surfactants(cosmetic)
Surfactants(cosmetic) Thickeners
Thickeners UV Filters
UV Filters Fertilizers
Fertilizers Soil Conditioners
Soil Conditioners Plant Growth Regulators
Plant Growth Regulators Animal Feed Additives
Animal Feed Additives Biostimulants
Biostimulants Pesticides (Herbicides, Insecticides, Fungicides)
Pesticides (Herbicides, Insecticides, Fungicides) Demulsifiers
Demulsifiers Hydraulic Fracturing Fluids
Hydraulic Fracturing Fluids Scale Inhibitors(oil)
Scale Inhibitors(oil) Surfactants(oil)
Surfactants(oil) Drilling Fluids
Drilling Fluids Dyes and Pigments
Dyes and Pigments Bleaching Agents
Bleaching Agents Softening Agents
Softening Agents Finishing Agents
Finishing Agents Antistatic Agents
Antistatic Agents Admixtures
Admixtures Waterproofing Agents
Waterproofing Agents Sealants and Adhesives
Sealants and Adhesives Curing Compounds
Curing Compounds Concrete Repair Chemicals
Concrete Repair Chemicals Anti-Corrosion Coatings
Anti-Corrosion Coatings Surfactants(cleaning)
Surfactants(cleaning) Builders
Builders Enzymes
Enzymes Solvents (Cleaning)
Solvents (Cleaning) Fragrances
Fragrances Electronic Chemicals
Electronic Chemicals Catalysts
Catalysts Lubricants
Lubricants Photographic Chemicals
Photographic Chemicals Refrigerants
Refrigerants Automotive chemicals
Automotive chemicals Pyrotechnic Chemicals
Pyrotechnic Chemicals Biodegradable Surfactants
Biodegradable Surfactants Bio-based Solvents
Bio-based Solvents Renewable Polymers
Renewable Polymers Carbon Capture Chemicals
Carbon Capture Chemicals Wastewater Treatment Chemicals
Wastewater Treatment Chemicals Pigments
Pigments Solvents(paint)
Solvents(paint) Specialty Coatings
Specialty Coatings Binders/Resins
Binders/Resins Additives
Additives Driers
Driers Anti-Corrosion Agents
Anti-Corrosion Agents Functional Coatings
Functional Coatings Application-Specific Coatings
Application-Specific Coatings Fresh Herbs
Fresh Herbs Ground Spices
Ground Spices Whole Spices
Whole Spices Spice Blends
Spice Blends Dried Herbs
Dried Herbs Leavening Agents
Leavening Agents Dough Conditioners
Dough Conditioners Flour Treatments
Flour Treatments Fat Replacers
Fat Replacers Decoratives
Decoratives Preservatives(baking)
Preservatives(baking) Plasticizers & Softeners
Plasticizers & Softeners Reinforcing Agents
Reinforcing Agents Adhesion Promoters
Adhesion Promoters Vulcanizing Agents
Vulcanizing Agents Antidegradants
Antidegradants Blowing Agents
Blowing Agents Fillers & Extenders
Fillers & Extenders Accelerators & Retarders
Accelerators & Retarders








![Nickel Sulphate [NiSO4(H2O)6] Extra Pure Amaris Chemicals](https://amarischemicalsolutions.com/wp-content/uploads/2025/08/Nickel-Sulphate-NiSO4H2O6-Extra-Pure-Amaris-Chemicals-430x430.png)















