“Aluminum Fine Powder” has been added to your cart. View cart
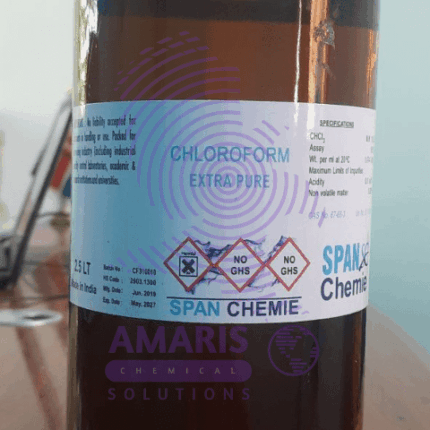
Chloroform 2.5litre extra pure
KSh4,500.00 Original price was: KSh4,500.00.KSh4,000.00Current price is: KSh4,000.00.
Cresol Red Indicator 25gm
KSh3,225.00 Original price was: KSh3,225.00.KSh2,200.00Current price is: KSh2,200.00.
Cobalt Chloride 100gm
KSh3,500.00 Original price was: KSh3,500.00.KSh3,000.00Current price is: KSh3,000.00.
SKU:
ACS91637CHEM0
Category: Analytical Reagents
Description
Cobalt Chloride is used to check for any water leakages in pipes and porcelains. This is done because cobalt chloride turns from blue to red when it reacts with water.
Reviews (0)
Be the first to review “Cobalt Chloride 100gm” Cancel reply
Shipping & Delivery
You may also like…
Cobalt Octoate 10% 200kg Drum
Cobalt octoate is a chemical compound that consists of cobalt ions (Co2+) bound to octoate ions (also known as octanoate ions, C8H15O2-). It is often used as a catalyst in various chemical reactions, particularly those involving the curing of unsaturated polyester resins and the polymerization of vinyl chloride. Cobalt octoate can also be used in the production of coatings, adhesives, and inks.
In its pure form, cobalt octoate appears as a dark blue liquid with a characteristic odor. It is soluble in a variety of organic solvents and is typically sold as a solution in mineral spirits or other solvents. The concentration of cobalt octoate in these solutions can vary depending on the intended use, with concentrations typically ranging from 1% to 12%.
Related products
Acetic Acid 2.5litre
Acetic acid is an organic acid with the chemical formula CH3COOH, also known as ethanoic acid. It is a colorless liquid with a pungent, sour taste and a distinctive vinegar-like odor. Acetic acid is an important industrial chemical used in the production of various products, including solvents, plastics, textiles, and food additives. It is also the main component of vinegar, which is commonly used as a condiment and preservative in cooking and food preparation.
Aluminum Ammonium Sulphate
Aluminum ammonium sulfate, also known as ammonium alum or just alum, is a chemical compound with the formula (NH4)Al(SO4)2·12H2O. It's a white crystalline solid commonly used in water purification, leather tanning, and as a mordant in dyeing textiles.
In water purification, alum acts as a coagulant to remove impurities by causing suspended particles to clump together, making it easier for filtration to remove them. In leather tanning, it helps to stabilize the leather by tightening the collagen fibers. And in dyeing textiles, alum helps the dye adhere to the fabric.
However, it's important to note that excessive exposure to aluminum compounds like alum can be harmful, so it's typically used with caution and proper safety measures.
Aluminum Carbonate 250g
Aluminum carbonate is a chemical compound with the formula Al2(CO3)3. It is a white, crystalline solid that is insoluble in water. Aluminum carbonate is not commonly encountered in pure form due to its high instability, especially in the presence of water and carbon dioxide. Instead, it tends to decompose into aluminum hydroxide and carbon dioxide when exposed to moisture or acidic conditions.
Aluminum Nitrate 500gm
Aluminum nitrate is a chemical compound with the formula Al(NO3)3. It's a salt composed of aluminum and nitrate ions. It's commonly encountered as a hydrate with varying numbers of water molecules associated with each aluminum nitrate formula unit. It's soluble in water and is often used in various industrial processes, including as a mordant in dyeing fabrics and in the production of aluminum oxide. Additionally, it's used in some chemical reactions and as a component in some types of rocket propellants.
Ammonium Dichromate 500gm
Anhydrous Aluminum Chloride
Anhydrous aluminum chloride, often represented as AlCl3, is a chemical compound composed of aluminum and chlorine. "Anhydrous" means it lacks water molecules in its structure. It's a white or pale yellow solid that is highly hygroscopic, meaning it readily absorbs moisture from the air. This property makes handling it a bit tricky since it can form a solution with water vapor in the air, turning into a fuming liquid.



















Reviews
There are no reviews yet.