![Ferric Chloride 98% 25kg [Anhydrous]](https://amarischemicalsolutions.com/wp-content/uploads/2025/05/Ferric-chloride98-25kg-amaris-chemicals-430x430.jpg)
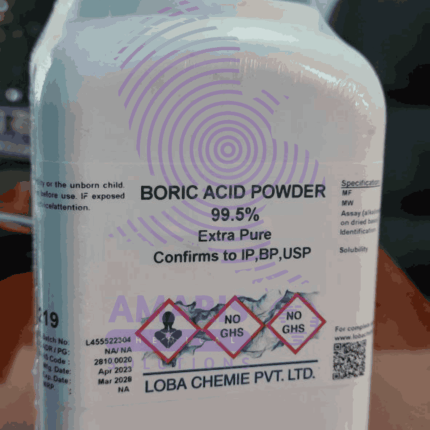
Boric Acid
$9,800.00 Original price was: $9,800.00.$8,900.00Current price is: $8,900.00.
Boric acid is a weak, water-soluble acid that occurs naturally in some minerals, volcanic waters, and hot springs. It is a white, odorless, and crystalline powder that is often used as an antiseptic, insecticide, flame retardant, and in various industrial applications. Boric acid is also commonly used in households as an eyewash, as a preservative for food and cosmetics, and as an ingredient in some laundry and cleaning products. It is considered a safe and effective substance when used properly, but can be toxic in high doses.
Boric acid Uses
Insecticide:
Boric acid is a natural and effective insecticide that can be used to control pests such as ants, cockroaches, termites, and silverfish.
Antiseptic:
Boric acid can be used as an antiseptic to treat minor cuts, burns, and fungal infections such as athlete’s foot and yeast infections.
Flame retardant:
Boric acid is often used as a flame retardant in textiles, plastics, and other materials.
Preservative:
Boric acid can be used as a preservative in food and cosmetics to prevent the growth of bacteria and fungi.
Industrial applications:
Boric acid is used in the production of fiberglass, ceramics, and other industrial materials.
Laundry and cleaning:
Boric acid can be used as a laundry booster and in cleaning products to remove stains and odors.
Eyewash:
Boric acid can be used as an eyewash to soothe irritated eyes.
| APPEARANCE |
Crystalline |
|---|---|
| AVAILABLE PACK SIZE |
25kg( Metal or Plastic Jerrycan/ Bucket, Bag, Box, Polythene bag, Carton bag) |
| COUNTRIES OF ORIGIN |
TURKEY |
1. Basic Identification Attributes
- Chemical Name: Boric acid (IUPAC: Trihydroxidoboron)
- CAS Number: 10043-35-3 / 11113-50-1 (orthoboric acid)
- HS Code: 2810.00.20 (Boric oxides & acids)
- Molecular Formula: H₃BO₃ or B(OH)₃
- Synonyms: Orthoboric acid, Boracic acid, Hydrogen borate
2. Physical & Chemical Properties
- Physical State: White crystalline powder or scales
- Color & Odor: Colorless/white; odorless
- Melting Point: 170.9°C (decomposes to metaboric acid)
- Boiling Point: 300°C (fully decomposes to B₂O₃)
- Density: 1.435 g/cm³ (15°C)
- Solubility:
- Water: 4.7 g/100mL (20°C), 27.5 g/100mL (100°C)
- Alcohol: Soluble in methanol (17.5 g/100mL)
- Glycerol: Highly soluble
- pH: 5.1 (0.1M aqueous solution)
- Refractive Index: 1.459
- Vapor Pressure: 6.7×10⁻⁶ mmHg (20°C)
3. Safety & Hazard Attributes
- GHS Classification:
- H360FD: May impair fertility/harm unborn child
- H319: Causes serious eye irritation
- NFPA Ratings: Health 1 | Flammability 0 | Reactivity 0
- Exposure Limits:
- OSHA PEL: 10 mg/m³ (total dust)
- ACGIH TLV: 2 mg/m³ (respirable fraction)
- Reactivity:
- Weak acid (pKa = 9.24)
- Incompatible with alkali metals, potassium
4. Storage & Handling
- Storage Conditions:
- Tightly sealed containers
- Ambient temperature (<25°C preferred)
- RH <50% (hygroscopic)
- Shelf Life: Indefinite if stored properly
- Handling PPE: Dust mask, gloves, eye protection
- Incompatibilities: Strong bases, alkali metals
5. Regulatory Status
- EPA: Registered as pesticide (PC Code: 011001)
- EU: CLP 08-1B (Reproductive toxicity)
- FDA: 21 CFR 175.105 (indirect food additive)
- Transport: Not classified as hazardous
6. Environmental & Health
- Ecotoxicity:
- LC50 (fish): 1,000-10,000 mg/L
- Moderately toxic to aquatic organisms
- Human Toxicity:
- Acute oral LD50: 2,660-3,450 mg/kg (rat)
- Chronic exposure affects reproduction
- Biodegradation: Not biodegradable
Personal Protection:
- Respiratory Protection: Use a dust mask (NIOSH-approved N95 or higher) if handling powder to avoid inhalation.
- Eye Protection: Wear safety goggles to prevent irritation.
- Skin Protection: Use gloves (nitrile or rubber) and protective clothing to minimize skin contact.
- General Hygiene: Avoid ingestion, inhalation, or prolonged exposure. Wash hands after handling.
Handling & Storage:
- Store in a cool, dry, well-ventilated area away from strong bases and alkali metals.
- Keep containers tightly sealed to prevent moisture absorption.
- Avoid generating dust; use local exhaust ventilation if handling large quantities.
- Do not eat, drink, or smoke near the chemical.
Spill Management:
- Sweep up spills (avoid creating dust) and dispose of in a sealed container.
- Wash contaminated surfaces with water.
Inhalation:
- Move to fresh air immediately.
- If breathing difficulties occur, seek medical attention.
Skin Contact:
- Remove contaminated clothing.
- Wash affected area with soap and water.
- If irritation persists, seek medical help.
Eye Contact:
- Rinse immediately with plenty of water for at least 15 minutes, lifting eyelids occasionally.
- Seek medical attention if irritation continues.
Ingestion:
- Do NOT induce vomiting.
- Rinse mouth and drink plenty of water.
- Seek immediate medical attention (boric acid can be toxic if ingested in large amounts).
Fire Hazard:
- Boric acid is non-flammable, but at high temperatures, it decomposes, releasing boron oxide fumes.
Extinguishing Media:
- Use water spray, dry chemical, CO₂, or foam for surrounding fires.
Protection for Firefighters:
- Wear self-contained breathing apparatus (SCBA) and full protective gear to avoid inhaling decomposition products.
- Special Notes: Cool containers exposed to fire with water to prevent rupture
You may also like…
Boric Acid 500gm
Related products
Acetaldehyde
- Chemical Structure: Acetaldehyde consists of two carbon atoms, one oxygen atom, and four hydrogen atoms. Its structure is CH3CHO, where the carbon atom in the middle is doubly bonded to an oxygen atom and singly bonded to a hydrogen atom and a methyl group (CH3).
- Occurrence: Acetaldehyde can be found naturally in various ripe fruits, coffee, and heated milk. It is also produced by the oxidation of ethanol (alcohol) by enzymes in the liver and other tissues in humans, making it an intermediate product in alcohol metabolism.


 Emollients
Emollients Humectants
Humectants UV Filters
UV Filters Surfactants (cosmetic)
Surfactants (cosmetic) Preservatives (cosmetic)
Preservatives (cosmetic) Fragrances and Essential Oils
Fragrances and Essential Oils Antioxidants (cosmetics)
Antioxidants (cosmetics)
 Solvents (lab)
Solvents (lab) Chromatography Chemicals
Chromatography Chemicals Microbiology and Cell Culture Reagents
Microbiology and Cell Culture Reagents Biochemical Reagents
Biochemical Reagents Inorganic and Organic Standards
Inorganic and Organic Standards Spectroscopy Reagents
Spectroscopy Reagents Molecular Biology Reagents
Molecular Biology Reagents
 Precious Metal Extraction Agents
Precious Metal Extraction Agents
 Plasticizers
Plasticizers Polymerization Initiators
Polymerization Initiators Stabilizers
Stabilizers Monomers
Monomers Fillers and Reinforcements
Fillers and Reinforcements Antioxidants (plastics)
Antioxidants (plastics) Colorants (plastic pigments,Dyes)
Colorants (plastic pigments,Dyes)
 Fertilizers
Fertilizers Plant Growth Regulators
Plant Growth Regulators Soil Conditioners
Soil Conditioners Animal Feed Additives
Animal Feed Additives Biostimulants
Biostimulants
 Dough Conditioners
Dough Conditioners Flour Treatments
Flour Treatments Fat Replacers
Fat Replacers Preservatives (baking)
Preservatives (baking)
 Surfactants (cleaning)
Surfactants (cleaning) Builders
Builders Bleaching Agents
Bleaching Agents Enzymes
Enzymes Solvents (cleaning)
Solvents (cleaning) Fragrances
Fragrances Disinfectant
Disinfectant Metal cleaning
Metal cleaning
 Binders/Resins
Binders/Resins Pigments
Pigments Solvents (paint)
Solvents (paint) Additives
Additives Driers
Driers Anti-Corrosion Agents
Anti-Corrosion Agents Specialty Coatings
Specialty Coatings Functional Coatings
Functional Coatings Application-Specific Coatings
Application-Specific Coatings
 Sealants and Adhesives
Sealants and Adhesives
 Biodegradable Surfactants
Biodegradable Surfactants Bio-based Solvents
Bio-based Solvents Renewable Polymers
Renewable Polymers Carbon Capture Chemicals
Carbon Capture Chemicals Wastewater Treatment Chemicals
Wastewater Treatment Chemicals
 Preservatives (food)
Preservatives (food) Flavor Enhancers
Flavor Enhancers Acidulants
Acidulants Sweeteners
Sweeteners Emulsifiers
Emulsifiers Antioxidants (food)
Antioxidants (food) Colorants (food)
Colorants (food) Nutrient Supplements
Nutrient Supplements Nutraceutical Ingredients
Nutraceutical Ingredients
 Fresh Herbs
Fresh Herbs Whole Spices
Whole Spices Ground Spices
Ground Spices Spice Blends
Spice Blends
 Surfactants(oil)
Surfactants(oil)
 Antibiotics
Antibiotics Active Pharmaceutical Ingredients
Active Pharmaceutical Ingredients Excipients
Excipients Vaccine Adjuvants
Vaccine Adjuvants Nutraceutical Ingredients
Nutraceutical Ingredients Solvents (pharmaceutical)
Solvents (pharmaceutical)
 Automotive chemicals
Automotive chemicals Pyrotechnic Chemicals
Pyrotechnic Chemicals


 Vulcanizing Agents
Vulcanizing Agents Accelerators & Retarders
Accelerators & Retarders Antidegradants
Antidegradants Reinforcing Agents
Reinforcing Agents Plasticizers & Softeners
Plasticizers & Softeners Fillers & Extenders
Fillers & Extenders Blowing Agents
Blowing Agents Adhesion Promoters
Adhesion Promoters