“Cobalt Octoate 10%” has been added to your cart. View cart
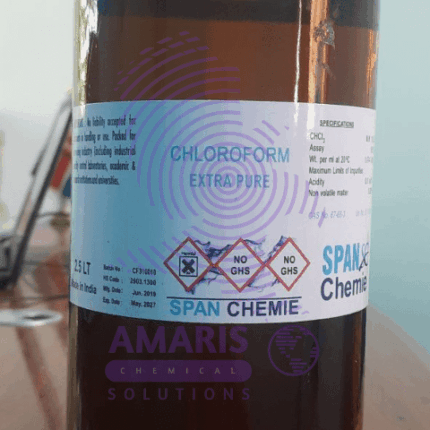
Chloroform 2.5litre extra pure
$4,500.00 Original price was: $4,500.00.$4,000.00Current price is: $4,000.00.
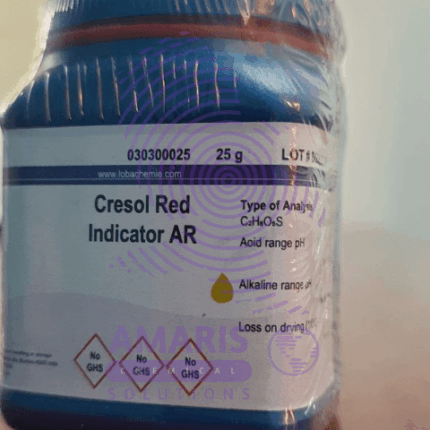
Cresol Red Indicator 25gm
$3,225.00 Original price was: $3,225.00.$2,200.00Current price is: $2,200.00.
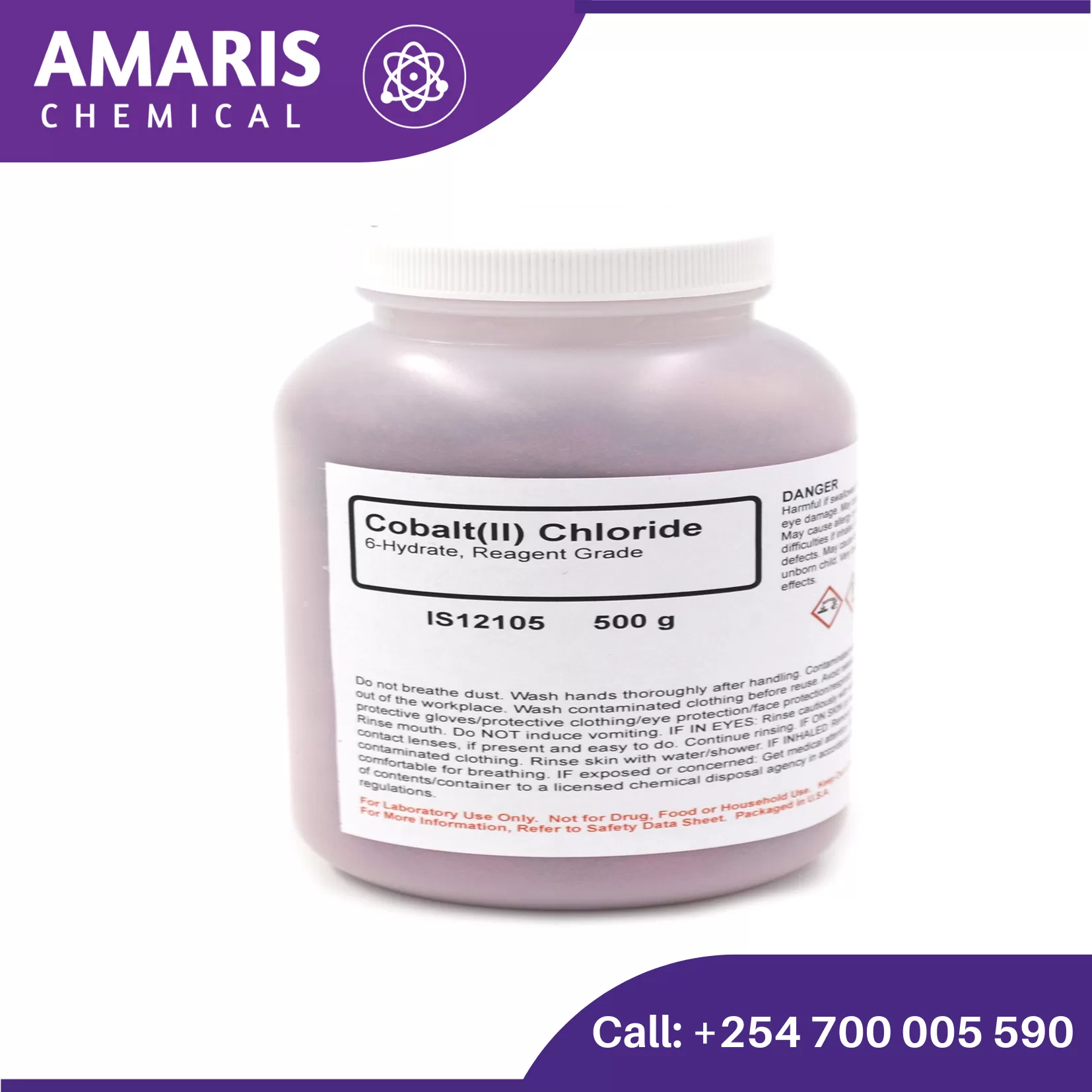
Cobalt Chloride 100gm
$3,500.00 Original price was: $3,500.00.$3,000.00Current price is: $3,000.00.
Whatsapp Order
SKU:
ACS91637CHEM0
Category: Analytical Reagents
Description
Cobalt Chloride is used to check for any water leakages in pipes and porcelains. This is done because cobalt chloride turns from blue to red when it reacts with water.
Reviews (0)
Be the first to review “Cobalt Chloride 100gm” Cancel reply
You may also like…
Cobalt Octoate 10%
Cobalt Octoate 10% is a chemical compound that consists of cobalt ions (Co2+) bound to octoate ions (also known as octanoate ions, C8H15O2-). It is often used as a catalyst in various chemical reactions, particularly those involving the curing of unsaturated polyester resins and the polymerization of vinyl chloride. Cobalt octoate can also be used in the production of coatings, adhesives, and inks.
In its pure form, cobalt octoate appears as a dark blue liquid with a characteristic odor. It is soluble in a variety of organic solvents and is typically sold as a solution in mineral spirits or other solvents. The concentration of cobalt octoate in these solutions can vary depending on the intended use, with concentrations typically ranging from 1% to 12%.
Related products
Aluminum Nitrate 500gm
Aluminum nitrate is a chemical compound with the formula Al(NO3)3. It's a salt composed of aluminum and nitrate ions. It's commonly encountered as a hydrate with varying numbers of water molecules associated with each aluminum nitrate formula unit. It's soluble in water and is often used in various industrial processes, including as a mordant in dyeing fabrics and in the production of aluminum oxide. Additionally, it's used in some chemical reactions and as a component in some types of rocket propellants.
Ammonium Dichromate 500gm
Ammonium Nitrate 500gm
Anhydrous Aluminum Chloride
Anhydrous aluminum chloride, often represented as AlCl3, is a chemical compound composed of aluminum and chlorine. "Anhydrous" means it lacks water molecules in its structure. It's a white or pale yellow solid that is highly hygroscopic, meaning it readily absorbs moisture from the air. This property makes handling it a bit tricky since it can form a solution with water vapor in the air, turning into a fuming liquid.
Sodium acetate Trihydrate
Sodium sulphate 25kg
Sodium Thiosulphate 25kg
Sodium thiosulfate (Na2S2O3) is an inorganic compound that is commonly used as a photographic fixer, as well as in medical and industrial applications. It is a white crystalline powder that is soluble in water and has a mild odor. In photography, sodium thiosulfate is used to remove unexposed silver halide from photographic prints and negatives, making the image permanent. In medicine, it is used as an antidote for cyanide poisoning, and in industrial applications, it is used as a reducing agent, a dechlorinating agent, and in water treatment processes.


 Emollients
Emollients Humectants
Humectants UV Filters
UV Filters Surfactants (cosmetic)
Surfactants (cosmetic) Preservatives (cosmetic)
Preservatives (cosmetic) Fragrances and Essential Oils
Fragrances and Essential Oils Antioxidants (cosmetics)
Antioxidants (cosmetics)
 Solvents (lab)
Solvents (lab) Chromatography Chemicals
Chromatography Chemicals Microbiology and Cell Culture Reagents
Microbiology and Cell Culture Reagents Biochemical Reagents
Biochemical Reagents Inorganic and Organic Standards
Inorganic and Organic Standards Spectroscopy Reagents
Spectroscopy Reagents Molecular Biology Reagents
Molecular Biology Reagents
 Precious Metal Extraction Agents
Precious Metal Extraction Agents
 Plasticizers
Plasticizers Polymerization Initiators
Polymerization Initiators Stabilizers
Stabilizers Monomers
Monomers Fillers and Reinforcements
Fillers and Reinforcements Antioxidants (plastics)
Antioxidants (plastics) Colorants (plastic pigments,Dyes)
Colorants (plastic pigments,Dyes)
 Fertilizers
Fertilizers Plant Growth Regulators
Plant Growth Regulators Soil Conditioners
Soil Conditioners Animal Feed Additives
Animal Feed Additives Biostimulants
Biostimulants
 Dough Conditioners
Dough Conditioners Flour Treatments
Flour Treatments Fat Replacers
Fat Replacers Preservatives (baking)
Preservatives (baking)
 Surfactants (cleaning)
Surfactants (cleaning) Builders
Builders Bleaching Agents
Bleaching Agents Enzymes
Enzymes Solvents (cleaning)
Solvents (cleaning) Fragrances
Fragrances Disinfectant
Disinfectant Metal cleaning
Metal cleaning
 Binders/Resins
Binders/Resins Pigments
Pigments Solvents (paint)
Solvents (paint) Additives
Additives Driers
Driers Anti-Corrosion Agents
Anti-Corrosion Agents Specialty Coatings
Specialty Coatings Functional Coatings
Functional Coatings Application-Specific Coatings
Application-Specific Coatings
 Sealants and Adhesives
Sealants and Adhesives
 Biodegradable Surfactants
Biodegradable Surfactants Bio-based Solvents
Bio-based Solvents Renewable Polymers
Renewable Polymers Carbon Capture Chemicals
Carbon Capture Chemicals Wastewater Treatment Chemicals
Wastewater Treatment Chemicals
 Preservatives (food)
Preservatives (food) Flavor Enhancers
Flavor Enhancers Acidulants
Acidulants Sweeteners
Sweeteners Emulsifiers
Emulsifiers Antioxidants (food)
Antioxidants (food) Colorants (food)
Colorants (food) Nutrient Supplements
Nutrient Supplements Nutraceutical Ingredients
Nutraceutical Ingredients
 Fresh Herbs
Fresh Herbs Whole Spices
Whole Spices Ground Spices
Ground Spices Spice Blends
Spice Blends
 Surfactants(oil)
Surfactants(oil)
 Antibiotics
Antibiotics Active Pharmaceutical Ingredients
Active Pharmaceutical Ingredients Excipients
Excipients Vaccine Adjuvants
Vaccine Adjuvants Nutraceutical Ingredients
Nutraceutical Ingredients Solvents (pharmaceutical)
Solvents (pharmaceutical)
 Automotive chemicals
Automotive chemicals Pyrotechnic Chemicals
Pyrotechnic Chemicals


 Vulcanizing Agents
Vulcanizing Agents Accelerators & Retarders
Accelerators & Retarders Antidegradants
Antidegradants Reinforcing Agents
Reinforcing Agents Plasticizers & Softeners
Plasticizers & Softeners Fillers & Extenders
Fillers & Extenders Blowing Agents
Blowing Agents Adhesion Promoters
Adhesion Promoters

























Reviews
There are no reviews yet.