Calcium Hypochlorite
$ 5.09 Original price was: $ 5.09.$ 4.97Current price is: $ 4.97.
Manganese Sulphate Monohydrate
Whatsapp Order
Manganese Sulphate Monohydrate is an inorganic chemical compound with the formula MnSO₄·H₂O. It appears as a pale pink crystalline powder and is highly soluble in water. It is primarily used as a source of manganese, an essential micronutrient, in fertilizers, animal feed, and industrial processes. This monohydrate form is the most stable and commonly used in agriculture and feed-grade applications. It also finds use in various chemical syntheses, electroplating, and as a reagent in laboratories.
Description
Table of Contents
Toggle
Manganese Sulphate Monohydrate
Primary Uses
- Agriculture and Fertilizer Industry
- Micronutrient Fertilizer: Used as a manganese supplement in soil to correct manganese deficiency in crops like cereals, fruits, vegetables, and legumes.
- Foliar Spray and Soil Application: Applied directly to plants or soil to improve photosynthesis, chlorophyll production, and enzyme activation.
- Component in NPK Blends: Included in multi-nutrient formulations for balanced crop nutrition.
- Animal Feed Industry
- Feed Additive: Serves as a manganese supplement in poultry, swine, cattle, and aquaculture feed to support bone development, enzyme function, and reproductive health.
- Premix Formulations: Used in vitamin-mineral premixes for livestock and pets.
- Chemical Industry
- Intermediate in Manganese Compound Production: Used in the manufacture of other manganese-based chemicals (e.g., manganese dioxide, manganese carbonate).
- Electroplating: Employed as an additive in electroplating baths for depositing manganese coatings.
- Battery Industry
- Raw Material in Battery Manufacturing: Used in the production of manganese dioxide for dry cell batteries (particularly alkaline and zinc-carbon batteries).
Secondary Uses
- Water Treatment
- Trace Element Supplementation: Occasionally used in treating drinking or industrial water where manganese is deficient or as part of a micronutrient mix.
- Laboratory Applications
- Analytical Reagent: Used in lab reagents and tests, including redox titrations and preparation of analytical manganese solutions.
- Textile and Dyeing Industry
- Catalyst/Modifier: Used in dyeing processes and textile treatments as a mordant or chemical modifier.
- Pharmaceuticals and Nutraceuticals
- Nutrient Source: May be used in trace amounts in vitamin or mineral formulations, although less common than food-grade alternatives.
KEY PRODUCT FEATURES
1. Basic Identification Attributes
- Chemical Name (IUPAC): Manganese(II) sulfate monohydrate
- Common/Trade Name: Manganese Sulphate Monohydrate
- CAS Number: 10034-96-5
- HS Code: 2833.29.30
- Synonyms: Manganous sulfate monohydrate; MnSO₄·H₂O; Pink salt of manganese; Manganese(II) sulfate hydrate
2. Physical & Chemical Properties
- Physical State: Crystalline solid
- Color & Odor: Pale pink; odorless
- Molecular Formula: MnSO₄·H₂O
- Molecular Weight: 169.02 g/mol
- Solubility: Highly soluble in water; insoluble in ethanol
- Density: ~2.95 g/cm³
- Melting Point: Decomposes at ~850 °C
- pH (5% Solution): ~4.0 – 6.0
- Stability: Stable under normal conditions
3. Safety & Hazard Attributes
- GHS Classification:
- Acute toxicity (oral) – Category 4
- Eye irritation – Category 2A
- Harmful to aquatic life – Category 2
- Toxicity:
- Harmful if swallowed in large quantities
- May cause irritation to eyes and respiratory tract
- Exposure Limits:
- ACGIH TLV (as Mn): 0.1 mg/m³ (inhalable)
- OSHA PEL (total Mn dust): 5 mg/m³ (ceiling limit)
4. Storage & Handling Attributes
- Storage Conditions:
- Store in a cool, dry, well-ventilated place
- Protect from moisture, as it may cake or dissolve
- Container Type:
- Packed in woven bags, drums, or HDPE-lined sacks
- Shelf Life:
- 2–3 years if stored in sealed packaging
- Handling Precautions:
- Avoid dust formation
- Use appropriate PPE (gloves, goggles, dust mask)
5. Regulatory & Compliance Attributes
- Compliance:
- Approved for use in agriculture and feed industries (FAO/WHO, AAFCO)
- REACH-registered and listed on TSCA inventory
- Must comply with heavy metal limits in food and feed use
- Labeling Requirements:
- Include hazard symbols for health and environmental risks
- State manganese content clearly for agricultural use
6. Environmental & Health Impact
- Biodegradability: Not applicable (inorganic compound)
- Ecotoxicity: Harmful to aquatic organisms at high concentrations
- Bioaccumulation: Not significant
- Carcinogenicity/Mutagenicity: Not classified as carcinogenic
- Soil Impact: Beneficial in appropriate doses; excess may inhibit other micronutrient uptake
SAFETY HANDLING PRECAUTIONS
Safety Handling Precautions
- PPE Required:
- Dust mask or respirator
- Safety goggles
- Protective gloves and clothing
- Handling Guidelines:
- Minimize dust generation
- Wash hands thoroughly after handling
- Storage Measures:
- Keep containers sealed and labeled
- Avoid contamination with food or feed not intended for fortification
First Aid Measures
- Inhalation: Move to fresh air; seek medical attention if irritation persists
- Skin Contact: Wash thoroughly with soap and water
- Eye Contact: Rinse immediately with water for at least 15 minutes
- Ingestion: Rinse mouth; seek medical advice if large amounts are ingested
Firefighting Measures
- Fire Hazards: Non-flammable
- Extinguishing Media: Use media suitable for surrounding materials (water, foam, dry chemical)
- Special Precautions: Avoid inhaling dust or fumes
- Hazardous Combustion Products: May release sulfur oxides and manganese oxides when decomposed at high temperatures
Related products
Acid Oil Soya
Acid Oil Soya is a byproduct derived from the refining of soybean oil. It is a dark-colored, free fatty acid-rich liquid containing mainly oleic and linoleic acids. Acid Oil Soya is commonly used in industrial applications such as soap making, animal feed, and as a raw material in the production of biodiesel, lubricants, and other chemicals.
Choline Chloride Powder
Choline Chloride Powder is a white to off-white crystalline powder containing 60% choline chloride, a quaternary ammonium salt essential as a nutrient in animal and poultry feed. It serves as a vital source of choline, an important component in fat metabolism, liver function, and cell membrane integrity. Produced through chemical synthesis, this powder form is highly soluble in water, making it easy to incorporate into feed premixes and supplements. Choline chloride is widely used in the agriculture industry to prevent choline deficiency, enhance growth performance, and improve overall health in livestock. Beyond animal nutrition, it finds applications in chemical manufacturing and pharmaceuticals.
Ferrous Gluconate
Ferrous Gluconate is an iron salt of gluconic acid, appearing as a pale greenish-blue or green crystalline powder or granules. It is widely used as a nutritional iron supplement and food additive due to its good bioavailability and relatively low toxicity compared to other iron salts. Ferrous Gluconate is water-soluble, providing a stable source of ferrous iron (Fe²⁺) that is easily absorbed in the gastrointestinal tract. This compound is commonly employed in pharmaceuticals, food fortification, and medical formulations to treat or prevent iron deficiency anemia. It is also used as a reducing agent and color stabilizer in various industrial applications.
Iodised Salt
Iodised Salt is table salt (sodium chloride) fortified with a small, controlled amount of iodine, typically in the form of potassium iodate or potassium iodide. It appears as fine white crystalline granules, odorless, and with a characteristic salty taste. The addition of iodine helps prevent iodine deficiency disorders (IDD) such as goiter, mental impairment, and developmental abnormalities. Iodised Salt is widely used in households, food processing, and animal nutrition to ensure adequate dietary iodine intake.
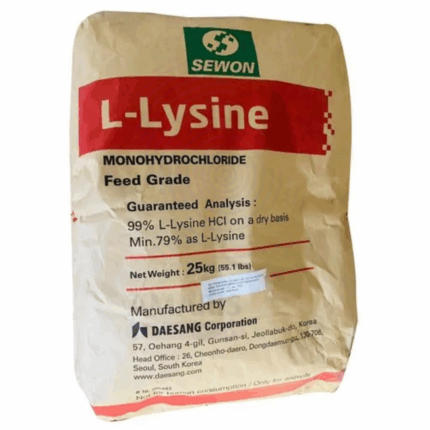
L-Lysine HCL Feed Grade
L-Lysine HCL Feed Grade is a highly pure form of the essential amino acid lysine combined with hydrochloric acid to improve its stability and solubility. It appears as a white crystalline powder and is widely used as a dietary supplement in animal feed to enhance growth, improve feed efficiency, and balance amino acid profiles. L-Lysine HCL is critical in poultry, swine, and aquaculture nutrition, helping to meet animals’ lysine requirements for protein synthesis and overall health.
Magnesium Oxide
Magnesium Oxide (MgO) is a white, odorless, alkaline earth metal oxide powder. It is produced by calcining magnesium carbonate or hydroxide at high temperatures, resulting in a fine, white powder with a high melting point. Magnesium Oxide is widely used for its refractory properties, chemical stability, and ability to neutralize acids. It serves important roles in pharmaceuticals, agriculture, environmental applications, and various industrial processes.
Magnesium Sulphate (Epsom Salt)
Magnesium Sulphate Epsom Salt, commonly known as Epsom Salt, is an inorganic salt composed of magnesium, sulfur, and oxygen with the formula MgSO₄. It typically appears as colorless or white crystalline granules and is highly soluble in water. In its heptahydrate form (MgSO₄·7H₂O), it is widely used in agriculture, pharmaceuticals, food, and industrial applications. Epsom Salt is valued for its muscle relaxant, laxative, and magnesium supplementation properties, as well as for its role in improving soil fertility and plant growth.
Tylosin Tartrate
Tylosin Tartrate is a water-soluble macrolide antibiotic derived from Streptomyces fradiae, primarily used in veterinary medicine. It is the tartrate salt form of tylosin, enhancing its solubility and making it ideal for administration via drinking water or injectable solutions. Tylosin Tartrate is active against a wide range of Gram-positive bacteria and some Gram-negative organisms, as well as Mycoplasma spp. It is particularly effective for treating respiratory, gastrointestinal, and reproductive infections in livestock and poultry.


 Preservatives(food)
Preservatives(food) Flavor Enhancers
Flavor Enhancers Acidulants
Acidulants Sweeteners
Sweeteners Antioxidants
Antioxidants Colorants(food)
Colorants(food) Nutraceutical Ingredients (food)
Nutraceutical Ingredients (food) Nutrient Supplements
Nutrient Supplements Emulsifiers
Emulsifiers
 Collectors
Collectors Dust Suppressants
Dust Suppressants Explosives and Blasting Agents
Explosives and Blasting Agents Flocculants and Coagulants
Flocculants and Coagulants Frothers
Frothers Leaching Agents
Leaching Agents pH Modifiers
pH Modifiers Precious Metal Extraction Agents
Precious Metal Extraction Agents
 Antioxidants(plastic)
Antioxidants(plastic) Colorants (Pigments, Dyes)
Colorants (Pigments, Dyes) Fillers and Reinforcements
Fillers and Reinforcements Flame Retardants
Flame Retardants Monomers
Monomers Plasticizers
Plasticizers Polymerization Initiators
Polymerization Initiators Stabilizers (UV, Heat)
Stabilizers (UV, Heat)
 Antifoaming Agents
Antifoaming Agents Chelating Agents
Chelating Agents Coagulants and Flocculants
Coagulants and Flocculants Corrosion Inhibitors
Corrosion Inhibitors Disinfectants and Biocides
Disinfectants and Biocides Oxidizing Agents
Oxidizing Agents pH Adjusters
pH Adjusters Scale Inhibitors( water)
Scale Inhibitors( water)
 Antioxidants(cosmetic)
Antioxidants(cosmetic) Emollients
Emollients Fragrances and Essential Oils
Fragrances and Essential Oils Humectants
Humectants Preservatives
Preservatives Surfactants(cosmetic)
Surfactants(cosmetic) Thickeners
Thickeners UV Filters
UV Filters
 Fertilizers
Fertilizers Soil Conditioners
Soil Conditioners Plant Growth Regulators
Plant Growth Regulators Animal Feed Additives
Animal Feed Additives Biostimulants
Biostimulants Pesticides (Herbicides, Insecticides, Fungicides)
Pesticides (Herbicides, Insecticides, Fungicides)
 Active Pharmaceutical Ingredients (APIs)
Active Pharmaceutical Ingredients (APIs) Excipients
Excipients Solvents(pharmaceutical)
Solvents(pharmaceutical) Antibiotics
Antibiotics Antiseptics and Disinfectants
Antiseptics and Disinfectants Vaccine Adjuvants
Vaccine Adjuvants Nutraceutical Ingredients (pharmaceutical)
Nutraceutical Ingredients (pharmaceutical) Analgesics & Antipyretics
Analgesics & Antipyretics
 Analytical Reagents
Analytical Reagents Solvents(lab)
Solvents(lab) Chromatography Chemicals
Chromatography Chemicals Spectroscopy Reagents
Spectroscopy Reagents microbiology-and-cell-culture-reagents
microbiology-and-cell-culture-reagents Molecular Biology Reagents
Molecular Biology Reagents Biochemical Reagents
Biochemical Reagents Inorganic and Organic Standards
Inorganic and Organic Standards Laboratory Safety Chemicals
Laboratory Safety Chemicals Specialty Laboratory Chemicals(Special Laboratory Equipment)
Specialty Laboratory Chemicals(Special Laboratory Equipment)
 Demulsifiers
Demulsifiers Hydraulic Fracturing Fluids
Hydraulic Fracturing Fluids Scale Inhibitors(oil)
Scale Inhibitors(oil) Surfactants(oil)
Surfactants(oil) Drilling Fluids
Drilling Fluids
 Dyes and Pigments
Dyes and Pigments Bleaching Agents
Bleaching Agents Softening Agents
Softening Agents Finishing Agents
Finishing Agents Antistatic Agents
Antistatic Agents
 Admixtures
Admixtures Waterproofing Agents
Waterproofing Agents Sealants and Adhesives
Sealants and Adhesives Curing Compounds
Curing Compounds Concrete Repair Chemicals
Concrete Repair Chemicals Anti-Corrosion Coatings
Anti-Corrosion Coatings
 Surfactants(cleaning)
Surfactants(cleaning) Builders
Builders Enzymes
Enzymes Solvents (Cleaning)
Solvents (Cleaning) Fragrances
Fragrances
 Electronic Chemicals
Electronic Chemicals Catalysts
Catalysts Lubricants
Lubricants Photographic Chemicals
Photographic Chemicals Refrigerants
Refrigerants Automotive chemicals
Automotive chemicals Pyrotechnic Chemicals
Pyrotechnic Chemicals
 Biodegradable Surfactants
Biodegradable Surfactants Bio-based Solvents
Bio-based Solvents Renewable Polymers
Renewable Polymers Carbon Capture Chemicals
Carbon Capture Chemicals Wastewater Treatment Chemicals
Wastewater Treatment Chemicals
 Pigments
Pigments Solvents(paint)
Solvents(paint) Specialty Coatings
Specialty Coatings Binders/Resins
Binders/Resins Additives
Additives Driers
Driers Anti-Corrosion Agents
Anti-Corrosion Agents Functional Coatings
Functional Coatings Application-Specific Coatings
Application-Specific Coatings
 Leavening Agents
Leavening Agents Dough Conditioners
Dough Conditioners Flour Treatments
Flour Treatments Fat Replacers
Fat Replacers Decoratives
Decoratives Preservatives(baking)
Preservatives(baking)
 Plasticizers & Softeners
Plasticizers & Softeners Reinforcing Agents
Reinforcing Agents Adhesion Promoters
Adhesion Promoters Vulcanizing Agents
Vulcanizing Agents Antidegradants
Antidegradants Blowing Agents
Blowing Agents Fillers & Extenders
Fillers & Extenders Accelerators & Retarders
Accelerators & Retarders