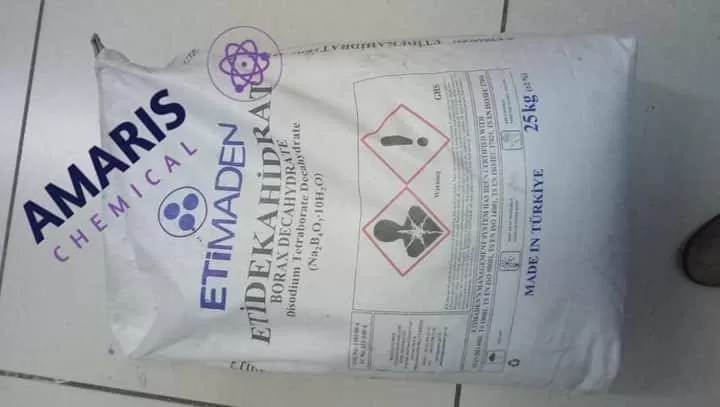
Borax Decahydrate
$8,500.00 Original price was: $8,500.00.$7,950.00Current price is: $7,950.00.
Borax decahydrate, also known as sodium borate, is a naturally occurring mineral composed of sodium, boron, oxygen, and water. It is a white, odorless powder that dissolves easily in water, and has a wide range of uses, including as a laundry detergent booster, a multipurpose cleaner, and as a component in the production of glass, ceramics, and enamel. Borax has antifungal and insecticidal properties and is also used in certain industrial applications such as in the production of fiberglass, as a flux in metallurgy, and as a fire retardant. It is considered safe when used as directed, but can be toxic if ingested in large quantities.
Borax decahydrate Uses
Laundry:
Borax can be added to laundry as a detergent booster to help remove stains and brighten clothes.
Cleaning:
Borax can be used as a general-purpose cleaner for surfaces such as countertops, sinks, and toilets.
Pest control:
Borax can be used as an insecticide to control ants, cockroaches, and other pests.
Cosmetics:
Borax can be used in cosmetics such as lotions and bath products to help stabilize and thicken them.
Glass and ceramics:
Borax is used in the production of glass and ceramics to improve their durability and resistance to heat.
Welding and metallurgy:
Borax is used as a flux in welding and metallurgy to help remove impurities from metals.
Fire retardant:
Borax can be used as a fire retardant in fabrics, wood, and other materials.
Agriculture:
Borax is used in agriculture as a micronutrient fertilizer for plants.
Photography:
Borax can be used in photography as a component of developer solutions.
| Weight | 25 kg |
|---|---|
| AVAILABLE PACK SIZE |
25kg( Metal or Plastic Jerrycan/ Bucket, Bag, Box, Polythene bag, Carton bag) |
1. Basic Identification Attributes
- Chemical Name: Sodium tetraborate decahydrate (IUPAC)
- CAS Number: 1303-96-4
- HS Code: 2840.19.00 (Borates)
- Molecular Formula: Na₂B₄O₇·10H₂O
- Synonyms: Sodium borate decahydrate, Disodium tetraborate decahydrate, "20 Mule Team" borax
2. Physical & Chemical Properties
- Physical State: White crystalline powder or granules
- Color & Odor: Colorless to white; odorless
- Boiling Point: 1575°C (anhydrous)
- Melting Point: 75°C (loses water), 743°C (anhydrous)
- Density: 1.73 g/cm³
- Solubility:
- Water: 31.7 g/L (20°C), 102 g/L (100°C)
- Organic solvents: Insoluble
- pH Level: 9.2-9.4 (1% aqueous solution)
- Vapor Pressure: Negligible
- Refractive Index: 1.472
- Decomposition: Begins at 75°C (loses water)
3. Safety & Hazard Attributes
- Hazard Class (GHS):
- H360FD: May damage fertility and the unborn child
- H319: Causes serious eye irritation
- NFPA Ratings: Health: 2 | Flammability: 0 | Reactivity: 0
- Exposure Limits:
- OSHA PEL: 10 mg/m³ (total dust)
- ACGIH TLV: 5 mg/m³ (inhalable fraction)
- Reactivity:
- Stable under normal conditions
- Reacts with strong acids
4. Storage & Handling Attributes
- Storage Conditions:
- Cool, dry, well-ventilated area
- Temperature: <30°C
- Relative humidity: <50%
- Incompatible Materials: Strong acids, metals (Al, Zn)
- Container Type: Plastic or lined metal containers
- Shelf Life: Indefinite if stored properly
- Special Handling:
- PPE: Dust mask, gloves, eye protection
- Avoid dust formation
5. Regulatory & Compliance Attributes
- Regulatory Status:
- EPA: Registered pesticide (insecticide)
- EU: Classified as Repr. 1B (fertility effects)
- REACH: Registered
- Hazard Symbols: GHS08 (Health hazard), GHS07 (Exclamation mark)
- Transportation:
- Not classified as dangerous goods for transport
- Waste Disposal:
- Dispose as hazardous waste (boron content)
6. Environmental & Health Impact
- Ecotoxicity:
- LC50 (fish): 1,000-10,000 mg/L
- Moderately toxic to aquatic organisms
- Persistence: Readily soluble, mobile in water
- Carcinogenicity:
- Not classified by IARC
- NTP: Not listed
- Biodegradability: Not biodegradable (inorganic)
Personal Protective Equipment (PPE)
- Respiratory Protection:Dust mask (N95) if handling powder in poorly ventilated areas
- Eye Protection:Safety goggles
- Skin Protection:Nitrile gloves, long sleeves (for prolonged exposure)
- General Hygiene:Wash hands after handling
Handling & Storage
- Storage:Keep in a cool, dry, well-ventilated area
- Incompatible Materials:Avoid strong acids (may release boric acid)
- Dust Control:Minimize airborne dust generation
- Housekeeping:Clean spills promptly to prevent slip hazards
Spill Management
- Small Spills:Sweep up carefully (avoid dust cloud)
- Large Spills:Dampen with water before cleanup
- Disposal:Follow local regulations (generally non-hazardous)
Inhalation (Dust Exposure)
- Move to fresh air
- If breathing difficulty occurs, seek medical attention
Skin Contact
- Wash thoroughly with soap and water
- Remove contaminated clothing
Eye Contact
- Flush with clean water for 15 minutes
- Seek medical attention if irritation persists
Ingestion
- Rinse mouth with water
- Do NOT induce vomiting
- Give 1-2 glasses of water to drink
- Seek medical advice (especially for large quantities)
Fire Characteristics
- Non-flammable(will not burn)
- No significant fire hazard
Exposure to Fire
- Decomposes at high temperatures (>75°C)
- May release boric oxide fumes (low toxicity)


 Emollients
Emollients Humectants
Humectants UV Filters
UV Filters Surfactants (cosmetic)
Surfactants (cosmetic) Preservatives (cosmetic)
Preservatives (cosmetic) Fragrances and Essential Oils
Fragrances and Essential Oils Antioxidants (cosmetics)
Antioxidants (cosmetics)
 Solvents (lab)
Solvents (lab) Chromatography Chemicals
Chromatography Chemicals Microbiology and Cell Culture Reagents
Microbiology and Cell Culture Reagents Biochemical Reagents
Biochemical Reagents Inorganic and Organic Standards
Inorganic and Organic Standards Spectroscopy Reagents
Spectroscopy Reagents Molecular Biology Reagents
Molecular Biology Reagents
 Precious Metal Extraction Agents
Precious Metal Extraction Agents
 Plasticizers
Plasticizers Polymerization Initiators
Polymerization Initiators Stabilizers
Stabilizers Monomers
Monomers Fillers and Reinforcements
Fillers and Reinforcements Antioxidants (plastics)
Antioxidants (plastics) Colorants (plastic pigments,Dyes)
Colorants (plastic pigments,Dyes)
 Fertilizers
Fertilizers Plant Growth Regulators
Plant Growth Regulators Soil Conditioners
Soil Conditioners Animal Feed Additives
Animal Feed Additives Biostimulants
Biostimulants
 Dough Conditioners
Dough Conditioners Flour Treatments
Flour Treatments Fat Replacers
Fat Replacers Preservatives (baking)
Preservatives (baking)
 Surfactants (cleaning)
Surfactants (cleaning) Builders
Builders Bleaching Agents
Bleaching Agents Enzymes
Enzymes Solvents (cleaning)
Solvents (cleaning) Fragrances
Fragrances Disinfectant
Disinfectant Metal cleaning
Metal cleaning
 Binders/Resins
Binders/Resins Pigments
Pigments Solvents (paint)
Solvents (paint) Additives
Additives Driers
Driers Anti-Corrosion Agents
Anti-Corrosion Agents Specialty Coatings
Specialty Coatings Functional Coatings
Functional Coatings Application-Specific Coatings
Application-Specific Coatings
 Sealants and Adhesives
Sealants and Adhesives
 Biodegradable Surfactants
Biodegradable Surfactants Bio-based Solvents
Bio-based Solvents Renewable Polymers
Renewable Polymers Carbon Capture Chemicals
Carbon Capture Chemicals Wastewater Treatment Chemicals
Wastewater Treatment Chemicals
 Preservatives (food)
Preservatives (food) Flavor Enhancers
Flavor Enhancers Acidulants
Acidulants Sweeteners
Sweeteners Emulsifiers
Emulsifiers Antioxidants (food)
Antioxidants (food) Colorants (food)
Colorants (food) Nutrient Supplements
Nutrient Supplements Nutraceutical Ingredients
Nutraceutical Ingredients
 Fresh Herbs
Fresh Herbs Whole Spices
Whole Spices Ground Spices
Ground Spices Spice Blends
Spice Blends
 Surfactants(oil)
Surfactants(oil)
 Antibiotics
Antibiotics Active Pharmaceutical Ingredients
Active Pharmaceutical Ingredients Excipients
Excipients Vaccine Adjuvants
Vaccine Adjuvants Nutraceutical Ingredients
Nutraceutical Ingredients Solvents (pharmaceutical)
Solvents (pharmaceutical)
 Automotive chemicals
Automotive chemicals Pyrotechnic Chemicals
Pyrotechnic Chemicals


 Vulcanizing Agents
Vulcanizing Agents Accelerators & Retarders
Accelerators & Retarders Antidegradants
Antidegradants Reinforcing Agents
Reinforcing Agents Plasticizers & Softeners
Plasticizers & Softeners Fillers & Extenders
Fillers & Extenders Blowing Agents
Blowing Agents Adhesion Promoters
Adhesion Promoters