Lead Iodide Extra Pure
Lead Iodide Extra Pure is a bright yellow crystalline compound with the formula PbI₂, known for its striking color and high chemical purity, making it ideal for laboratory and research use. It is commonly used in materials science, especially in the fabrication of perovskite solar cells and semiconductors, due to its optical properties and ability to form thin films. In academic research, it plays a role in crystal growth experiments, and its distinct color change upon heating serves as a classic demonstration in chemistry education. Owing to its lead content, appropriate handling precautions and environmental safeguards are essential when working with this compound.
Lead Monoxide Extra Pure
Lead Monoxide Extra Pure, also known as litharge (PbO), is a fine yellow or reddish powder widely used in analytical chemistry and industrial processes. Its high purity makes it suitable for laboratory-grade reactions, especially in the manufacture of lead-based compounds, ceramics, glass, and batteries. In the ceramics and glass industries, it enhances the brilliance and stability of glazes and optical glass. In metallurgy, it is used as a fluxing agent during refining. Due to its toxicity and lead content, it requires strict safety handling procedures and controlled storage conditions to prevent contamination or exposure.
Litmus Granular Extra Pure
Litmus Granular Extra Pure is a premium-grade pH indicator widely used in laboratories for acid-base testing and educational demonstrations. Derived from natural sources like lichens, this granular form offers a stable, easy-to-handle format suitable for preparing litmus solutions or impregnating test papers. In acidic environments, it turns red, while in alkaline conditions, it turns blue, providing a clear visual response. Its high purity ensures reliable and repeatable results across various applications in chemistry, biology, and environmental testing. Store in a tightly sealed container away from moisture and direct sunlight to preserve its effectiveness.
Litmus Solution Extra Pure
Litmus Solution Extra Pure is a high-quality, ready-to-use pH indicator solution designed for precise acid-base testing in laboratories and educational settings. Made from purified natural litmus dye, it changes color distinctly—red in acidic solutions and blue in alkaline solutions—making it an essential tool for quick and accurate pH assessments. Its extra pure formulation ensures consistent performance, clear color transitions, and reliability in analytical chemistry, biology experiments, and environmental monitoring. Ideal for titrations and classroom demonstrations, this solution should be stored in a cool, dark place to maintain stability and color accuracy.
Lugols Iodine Extra Pure
Lugols Iodine Extra Pure is a superior-grade aqueous solution composed of elemental iodine and potassium iodide, known for its wide-ranging applications in laboratories, microscopy, and medical diagnostics. Valued for its strong staining properties, it is commonly used in biology to identify starch in plant cells and as a general-purpose cellular stain to enhance visibility under a microscope. Its extra pure formulation ensures high clarity, minimal impurities, and consistent results in sensitive applications. Additionally, it plays a role in preparing specimens, sterilization procedures, and certain medical antiseptic practices. Store tightly sealed, away from light and heat, to preserve potency.
Lycopodium Powder Extra Pure
Lycopodium Powder Extra Pure is a fine, yellowish powder derived from the spores of club moss plants, prized for its exceptional purity and versatility in both scientific and industrial applications. In laboratories, it is frequently used to demonstrate Brownian motion, study spore dispersion, or simulate particulate flow in airflow experiments. Due to its high flammability, Lycopodium powder also serves as a dramatic visual aid in physics demonstrations involving flame propagation or combustion. Outside the lab, it has niche applications in pharmaceuticals, cosmetics, and even pyrotechnics. It should be handled with care and stored in a dry, cool, and non-igniting environment.
Magnesium Ascorbyl Phosphate Extra Pure
Magnesium Ascorbyl Phosphate Extra Pure is a highly stable, water-soluble derivative of Vitamin C, widely appreciated for its exceptional antioxidant properties and gentle skin compatibility. Commonly used in cosmetic and dermatological formulations, it helps brighten the skin, reduce hyperpigmentation, and support collagen synthesis—making it a valuable ingredient in anti-aging and skin-repair treatments. Its stability against oxidation allows it to retain potency longer than pure ascorbic acid, especially in aqueous solutions. With pharmaceutical and cosmetic grade purity, this compound is ideal for use in serums, lotions, and creams targeting a healthy, radiant complexion.
Magnesium Chloride Extra Pure
Magnesium Chloride Extra Pure is a highly soluble, refined chemical compound commonly used for its excellent hygroscopic and electrolyte properties. In laboratory and analytical settings, it is valued for preparing magnesium-based solutions and as a reagent in various chemical reactions. In pharmaceuticals, it serves as a magnesium supplement, aiding in nerve function and muscle health. It is also used in cosmetics and personal care products for its skin-conditioning benefits. Additionally, it finds applications in textile processing, dust control, and de-icing formulations, where its purity ensures minimal impurities in sensitive applications.
Magnesium Hydroxide Heavy Extra Pure
Magnesium Hydroxide Heavy Extra Pure is a high-purity, dense white powder widely utilized for its antacid and absorbent properties. In pharmaceutical formulations, it acts as a gentle laxative and an effective antacid to neutralize stomach acid. Due to its high purity, it is also used in laboratory experiments and analytical chemistry where precise reactions are essential. In cosmetic and personal care products, it functions as a pH adjuster and an absorbent in creams, lotions, and deodorants. Additionally, it serves roles in wastewater treatment, flame retardants, and ceramic manufacturing, offering consistent performance and low impurity content.
Magnesium Nitrate Hexahydrate Extra Pure
Magnesium Nitrate Hexahydrate Extra Pure is a crystalline compound known for its high solubility in water and exceptional purity, making it suitable for both laboratory and industrial applications. In analytical chemistry, it is commonly used as a reagent for preparing standard solutions and as a source of magnesium ions in various experimental procedures. Its oxidizing properties make it valuable in pyrotechnics and fertilizer formulations, where it provides essential nutrients for plant growth. This compound is also employed in ceramic production, textile treatments, and catalyst preparations, where a consistent, high-quality nitrate source is required. Its extra pure grade ensures minimal contamination, essential for high-precision work.
Magnesium Oxide Heavy Extra Pure
Magnesium Oxide Heavy Extra Pure is a high-purity, dense white powder prized for its excellent thermal stability, high melting point, and chemical inertness. It is widely used in laboratory applications as a standard reagent, pH adjuster, and desiccant. In pharmaceuticals, it functions as an antacid and a magnesium supplement, while in industrial processes, it is essential for producing refractory materials, ceramics, and insulating products due to its high heat resistance. The "heavy" form refers to its higher bulk density, making it particularly useful in applications where volume constraints are critical. Its extra pure grade ensures it meets the stringent demands of high-precision research and formulation work.
Magnesium Oxide Light Powder Extra Pure
Magnesium Oxide Light Powder Extra Pure is a fine, white, odorless powder with a lower bulk density compared to the heavy grade, offering high surface area and excellent reactivity. It is widely used in analytical laboratories, pharmaceuticals, and cosmetics due to its purity and gentle chemical behavior. In pharmaceutical formulations, it acts as an antacid and magnesium supplement, while in cosmetics, it is used for its absorbent and pH-adjusting properties. Its light, fluffy texture also makes it ideal for use in rubber, plastics, and coatings as a filler, stabilizer, or neutralizing agent. The extra pure grade ensures consistent quality for precise and sensitive applications.
Malachite Green Extra Pure
Malachite Green Extra Pure is a synthetic dye of exceptional purity, recognized for its intense green coloration and versatile laboratory uses. It is commonly used as a biological stain, especially in microbiology for identifying and differentiating bacterial species, and in histology for staining cell structures. Additionally, Malachite Green serves as an effective fungicide and antiparasitic agent in aquaculture and laboratory testing. Its high purity ensures reliable performance in analytical applications, including redox titrations and as a pH indicator in specific chemical reactions. Its vivid color and chemical stability make it a valuable reagent in both teaching and professional research settings.
Maleic Acid Extra Pure
Maleic Acid Extra Pure is a high-purity organic compound valued for its strong acidic properties and wide range of laboratory and industrial applications. It appears as a white crystalline solid and is primarily used in organic synthesis, particularly in the production of polymers, resins, and plasticizers. In research laboratories, Maleic Acid serves as a buffering agent, a precursor to fumaric acid, and an intermediate in various chemical reactions. Its excellent solubility in water and compatibility with other reactants make it especially useful in biochemical and analytical procedures. The extra pure grade ensures minimal contamination, providing reliable results in sensitive experiments and high-precision manufacturing processes.
Maltose Extra Pure
Maltose Extra Pure is a disaccharide composed of two glucose units, commonly known as malt sugar. This extra pure grade ensures exceptional quality and consistency, making it ideal for use in biochemical research, fermentation studies, and laboratory analysis. In the food industry, maltose is valued for its mild sweetness and is often used in brewing, baking, and confectionery to promote fermentation and enhance flavor. It also plays a role in energy metabolism studies and is frequently used as a carbon source in microbiological media. Its stability and solubility in water make it easy to incorporate into various formulations, offering a dependable ingredient for scientific and industrial use where high purity is essential.
Manganese Dioxide Extra Pure
Manganese Dioxide Extra Pure is a high-purity, dark brown or black powder widely used in laboratories and industrial processes for its strong oxidizing properties. In analytical chemistry, it serves as an effective oxidizing agent and is often involved in the decomposition of hydrogen peroxide and other redox reactions. It is also used in the preparation of oxygen and chlorine gases in laboratory settings. Beyond the lab, manganese dioxide plays a crucial role in battery manufacturing, particularly in dry cell batteries, and in ceramics and glass production for coloring and decolorizing. Its consistent purity makes it ideal for sensitive experiments and formulations.
Manganese Sulphate Extra Pure
Manganese Sulphate Extra Pure is a highly refined, pale pink crystalline compound widely valued for its use in both laboratory and industrial applications. In the lab, it serves as a reliable source of manganese ions in analytical chemistry, synthesis of manganese-based catalysts, and as a micronutrient in biological research. Industrially, it plays a crucial role in fertilizers, where it addresses manganese deficiency in crops, and in animal feed formulations. Due to its high solubility and purity, this compound is also used in electroplating, ceramics, and pigment production, making it a versatile and essential chemical in many scientific and manufacturing settings.
Manganous Dihydrogen Phosphate Extra Pure
Manganous Dihydrogen Phosphate Extra Pure is a high-purity, white to pale pink crystalline compound composed of manganese and phosphate ions. It is primarily used in analytical chemistry, research laboratories, and specialized industrial processes where a precise and reliable manganese source is required. This compound is valued for its solubility in water and its role as a reagent in studies involving nutrient uptake, coordination chemistry, and phosphate-related reactions. Additionally, it finds niche applications in fertilizers and micronutrient formulations, particularly in controlled agricultural studies. Its extra pure grade ensures minimal contamination, making it suitable for sensitive experiments and formulations.
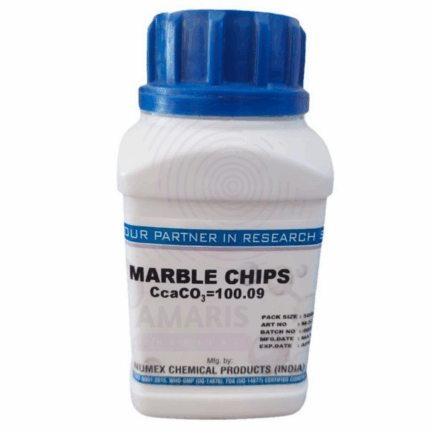
Marble Chips Extra Pure
Marble Chips Extra Pure are high-grade, naturally occurring calcium carbonate fragments that have been carefully processed to ensure exceptional purity. These white or off-white chips are widely used in laboratory experiments, especially in demonstrations involving acid-carbonate reactions where the release of carbon dioxide is observed. Beyond educational and research use, they also serve as a buffering agent, filtration medium, and mild abrasive in various industrial applications. Due to their chemical stability and high calcium content, marble chips are ideal for use in controlled environments where contaminants must be minimized.
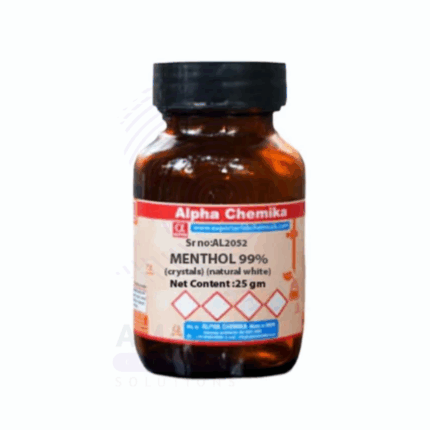
Menthol Crystals Extra Pure
Menthol Crystals Extra Pure are colorless to white, needle-like crystals derived from peppermint or other mint oils and refined to the highest purity. Known for their strong, refreshing minty aroma, they are widely used in pharmaceutical, cosmetic, and personal care formulations. These crystals provide a cooling sensation on the skin and mucous membranes, making them a common ingredient in balms, liniments, throat lozenges, and inhalants. In laboratories, menthol crystals are also used in sensory studies and product development. Their purity ensures consistent performance and minimal interference in sensitive formulations.
Methanol Extra Pure
Methanol Extra Pure is a clear, colorless, and highly volatile liquid with a mild alcohol-like odor, refined to an exceptional level of purity. It is widely used as a solvent in laboratories and industrial settings due to its excellent miscibility with water and many organic compounds. Methanol serves as a key reagent in chemical synthesis, particularly in the production of formaldehyde, methyl esters, and biodiesel. In analytical chemistry, it’s used for chromatography and spectroscopic applications where high purity is essential. Its rapid evaporation rate and strong solvency make it ideal for cleaning delicate equipment and preparing lab samples.
Methoxy Benzophenone Sulfonic Acid Extra Pure
Methoxy Benzophenone Sulfonic Acid Extra Pure is a high-purity, water-soluble organic compound primarily used as a UV filter in cosmetic and pharmaceutical formulations. This compound is valued for its ability to absorb harmful ultraviolet radiation, particularly in the UVB range, making it a key ingredient in sunscreens, lotions, and other personal care products requiring photo-stability. In laboratory settings, it is employed in research involving photochemistry and polymer stabilization. Its sulfonic acid group enhances water solubility, while the methoxy and benzophenone components provide strong UV-absorbing characteristics, ensuring effective and consistent performance in formulations demanding high safety and efficacy standards.
Methyl Orange Disodium Salt Extra Pure
Methyl Orange Disodium Salt Extra Pure is a high-purity pH indicator commonly used in titrations and analytical chemistry to determine acidity levels. It exhibits a sharp color change from red in acidic environments to yellow in alkaline conditions, typically within the pH range of 3.1 to 4.4. This disodium salt form ensures excellent solubility in water, making it especially reliable for use in aqueous solutions. Its stability and sensitivity make it ideal for laboratory applications involving strong acid–base reactions, particularly in titrations of mineral acids. Methyl Orange Disodium Salt Extra Pure is a trusted choice for consistent and accurate results in both educational and professional lab settings.
Methyl Orange Solutions Extra Pure
Methyl Orange Solutions Extra Pure is a ready-to-use, high-grade pH indicator solution widely utilized in acid-base titrations and general analytical chemistry. Known for its distinct and sharp color transition—from red in acidic environments to yellow in alkaline conditions—it provides rapid and clear visual cues for end-point detection. This extra pure formulation ensures maximum accuracy, reliability, and reproducibility in laboratory settings. It is especially effective in titrations involving strong acids and weak bases, commonly used in educational, research, and industrial labs. The solution is precisely prepared to standardized concentrations, eliminating the need for dilution or mixing, and is ideal for professionals seeking precision and ease of use.
Methyl Paraben Extra Pure
Methyl Paraben Extra Pure is a high-purity chemical widely used as a preservative in pharmaceuticals, cosmetics, personal care products, and food applications. It is valued for its strong antimicrobial and antifungal properties, helping to extend the shelf life of various formulations by preventing the growth of bacteria and mold. In laboratories, it is commonly employed in microbiological media and analytical standards. Its excellent stability across a broad pH range, combined with low toxicity and high efficacy even at low concentrations, makes it a preferred choice in sensitive applications. This extra pure grade ensures consistent quality and performance for critical uses.
Methyl Red Extra Pure
Methyl Red Extra Pure is a high-grade pH indicator commonly used in analytical chemistry, especially in titration processes to identify the endpoint between acidic and basic solutions. It exhibits a clear color change from red in acidic environments (pH below 4.4) to yellow in alkaline conditions (pH above 6.2), making it ideal for monitoring pH transitions in laboratory procedures. Beyond titrations, it is also used in microbiological media to distinguish between types of bacterial metabolism. This extra pure quality ensures excellent sensitivity, precision, and reliability in both educational and professional scientific settings.
Methyl Red Solution Extra Pure
Methyl Red Solution Extra Pure is a ready-to-use, high-purity pH indicator solution widely used in laboratories for acid-base titrations and biochemical assays. It changes color from red at pH below 4.4 to yellow above pH 6.2, making it highly effective in detecting pH shifts with clear visual transitions. This solution is particularly valuable in analytical chemistry, microbiology (such as the methyl red test in bacterial identification), and educational experiments. Its extra pure grade ensures consistent performance, high accuracy, and minimal impurities, making it suitable for precise scientific applications.
Methylene Blue Powder Extra Pure
Methylene Blue Powder Extra Pure is a high-grade dye with diverse laboratory and industrial applications. It is widely used as a biological stain in microscopy for highlighting cellular structures such as nuclei, bacteria, and parasites, particularly in blood smear and bacteriological studies. In chemistry, it serves as a redox indicator due to its distinct color change properties, and in aquaculture, it acts as a treatment for fungal and bacterial infections in fish. Its extra pure formulation ensures maximum staining clarity, minimal contamination, and reliable results in research, diagnostics, and educational settings.
Methylene Blue Solution Extra Pure
Methylene Blue Solution Extra Pure is a ready-to-use aqueous formulation of high-purity methylene blue dye, ideal for laboratory, biological, and analytical applications. It is commonly employed as a vital stain in microscopy to highlight cellular components such as nuclei and mitochondria, especially in live or fixed cells. This solution also functions as a redox indicator in various chemical experiments and is used in microbiology for bacterial identification and differentiation. With its extra pure quality, the solution delivers consistent and precise staining results, making it a reliable tool for research, diagnostics, and teaching laboratories.
Millions Reagent Extra Pure
Millions Reagent Extra Pure is a specialized analytical solution primarily used to detect phenolic amino acids, particularly tyrosine, in proteins. It functions by producing a reddish-brown coloration when heated with compounds containing phenol groups, making it highly valuable in biochemical testing and protein analysis. This reagent is a mixture of mercury(I) and mercury(II) nitrates in nitric acid, and its high purity ensures sharp, interference-free results, especially in sensitive laboratory experiments or educational demonstrations.
Due to its toxic and corrosive nature, proper handling is essential. It must be used in a fume hood with appropriate personal protective equipment such as gloves, goggles, and lab coats. Millon’s Reagent remains a reliable and well-established tool for identifying proteins with tyrosine residues and is commonly found in chemical, clinical, and educational labs.
Mimosa Powder Extra Pure
Mimosa Powder Extra Pure is a finely ground, high-purity botanical extract derived from the bark or leaves of Acacia spp. (commonly known as mimosa). This extra pure grade is meticulously processed to retain key phytochemicals—such as tannins, flavonoids, and phenolic compounds—in highly consistent concentrations. In laboratory settings, it is prized for use in phytochemical analysis, antioxidant testing, and evaluation of anti-inflammatory or antimicrobial activity.
With minimal impurities, Mimosa Powder is well-suited for biochemical assays, formulation trials, and comparative research on natural extracts. It may also be used in cosmetic formulations, nutraceutical studies, or dietary supplement development, where standardization and safety are critical. Proper storage in cool, dry, and dark conditions is recommended to maintain potency and prevent degradation.
Mineral Lick Rock Salt Extra Pure
Mineral Lick Rock Salt Extra Pure is a high-purity, natural crystalline salt product specially processed for scientific, veterinary, and nutritional applications. Composed primarily of sodium chloride (NaCl), this form of rock salt may also contain trace essential minerals such as calcium, magnesium, and potassium in controlled amounts. Its extra pure grade ensures minimal contaminants, making it ideal for controlled laboratory experiments, animal nutrition studies, and formulation testing where purity and consistency are critical.
In agricultural research and veterinary science, Mineral Lick is commonly used to study electrolyte balance, mineral supplementation, and livestock behavior related to mineral intake. The solid, stable nature of the product allows for long shelf life and ease of handling, and it may also be used in comparative tests involving natural versus synthetic mineral supplements. For best results, it should be stored in a dry, cool place away from moisture.
Naphthalene Balls Extra Pure
Naphthalene Balls Extra Pure are solid, white crystalline spheres composed of high-purity naphthalene (C₁₀H₈), refined for laboratory, industrial, and specialized household uses. Known for their strong, distinctive odor, these balls sublimate—changing directly from solid to vapor—making them exceptionally effective as fumigants and insect repellents, particularly against moths, silverfish, and mold in stored fabrics and books.
In laboratory settings, extra pure naphthalene balls are often used as a standard hydrocarbon compound for melting point determination, sublimation studies, and chemical synthesis. Their consistent purity and volatile nature also make them suitable for organic chemistry demonstrations and reactions. For safety, they should be handled in well-ventilated areas, kept away from flames or heat sources, and stored in airtight containers to minimize inhalation and fire risks.
Naphthalene Powder Extra Pure
Naphthalene Powder Extra Pure is a fine, white to pale crystalline powder composed of high-purity naphthalene (C₁₀H₈), valued for its consistent quality and volatile aromatic properties. This form is particularly suitable for laboratory use, where controlled dosing and faster sublimation are required. The powder is widely utilized in organic synthesis, standardization experiments, and melting point calibration, owing to its stable molecular structure and high sublimation rate.
In industrial and technical contexts, naphthalene powder serves as a precursor in dye manufacturing, plasticizer production, and pesticide formulation. It is also used as a fumigant for controlling pests in storage environments, similar to its use in ball form, but with faster release due to the increased surface area. Despite its utility, the powder must be handled with care—used in well-ventilated areas, kept away from ignition sources, and stored in sealed containers—due to its flammability and potential health effects from prolonged inhalation.
Neutral Red Solution Extra Pure
Neutral Red Solution Extra Pure is a high-purity, ready-to-use solution of the pH indicator dye Neutral Red, widely employed in biological, biochemical, and analytical laboratories. Known for its distinct color change—red in acidic environments and yellow in alkaline—it is commonly used to stain lysosomes in viable cells, making it invaluable in cell viability assays, cytotoxicity studies, and microscopic cell structure investigations.
This Extra Pure grade ensures excellent clarity and precision in results, especially in sensitive microbiological and histological procedures. It is frequently used in conjunction with culture media to observe metabolic activities and in vital staining techniques to differentiate between live and dead cells. Proper handling involves storing it in tightly sealed amber containers away from light and heat to maintain its stability and effectiveness.
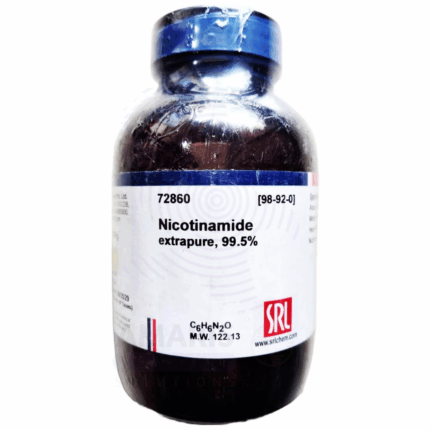
Nicotinamide Extra Pure
Nicotinamide Extra Pure is a refined, high-purity form of vitamin B3, widely recognized for its essential role in human and animal health. It serves as a vital component in the synthesis of NAD⁺ and NADP⁺, coenzymes that are fundamental to numerous metabolic and enzymatic reactions in living cells. In laboratory settings, it is frequently used in biochemical research, cell culture media, and vitamin assays due to its stable and bioactive nature.
In pharmaceutical applications, nicotinamide is commonly used in the formulation of supplements and dermatological treatments, where it supports skin barrier function, reduces inflammation, and helps alleviate acne and hyperpigmentation. Its presence in cosmetic products is valued for promoting smoother, more even-toned skin. The Extra Pure grade guarantees minimal impurities, making it ideal for sensitive applications in health care, research, and personal care products. It should be stored in a cool, dry environment away from light to maintain its integrity.
Nitric Acid Extra pure
Nitric Acid Extra pure is a high-purity, concentrated mineral acid supplied in a secure 2.5-litre packaging, ideal for laboratory and industrial applications requiring precision and consistency. Known for its strong oxidizing properties, nitric acid (HNO₃) is widely used in analytical chemistry, metal etching, sample digestion, and nitrate salt preparation.
This Extra Pure grade ensures minimal contaminants, making it especially suitable for trace analysis, high-purity synthesis, and quality control laboratories. Its sharp, acrid odor and highly corrosive nature demand careful handling with proper personal protective equipment (PPE), including acid-resistant gloves, goggles, and fume hoods. It must be stored in tightly sealed, compatible containers away from organic materials, bases, and flammable substances. This reagent is essential in both academic research and industrial processes involving nitration reactions, fertilizer formulation, and explosives development.
Octyl Methoxycinnamate Extra Pure
Octyl Methoxycinnamate Extra Pure is a high-purity organic compound widely used as a UV-B filter in sunscreen formulations and personal care products. Known chemically as Ethylhexyl Methoxycinnamate, it functions by absorbing ultraviolet light in the 280–320 nm range, thereby protecting skin from sunburn and photoaging.
In its Extra Pure form, OMC offers exceptional quality for cosmetic-grade applications, pharmaceutical formulations, and research requiring stringent purity levels. It is typically a clear, oily liquid with good solubility in oils and alcohols, making it ideal for incorporation into emulsions, creams, and lotions. Aside from its cosmetic uses, it is occasionally used in plastic and polymer stabilization where UV protection is needed. It should be stored in cool, dark conditions to maintain its stability and efficacy.
Oxalic Acid Extra Pure
Oxalic Acid Extra Pure is a high-purity, crystalline organic compound known for its strong reducing and chelating properties. Appearing as a white, odorless solid, it is highly soluble in water and widely utilized in both laboratory and industrial settings.
This Extra Pure grade ensures reliable performance in analytical chemistry, metal cleaning, rust removal, and rare earth element processing. In the laboratory, it is commonly used to precipitate calcium ions and to prepare standard solutions for titration. In industries, Oxalic Acid is valued for its effectiveness in bleaching wood, cleaning marble, and removing iron stains from surfaces and textiles. Due to its toxicity and corrosiveness, it must be handled with appropriate personal protective equipment and stored securely in a cool, dry, well-ventilated area.


 Preservatives(food)
Preservatives(food) Flavor Enhancers
Flavor Enhancers Acidulants
Acidulants Sweeteners
Sweeteners Antioxidants
Antioxidants Colorants(food)
Colorants(food) Nutraceutical Ingredients (food)
Nutraceutical Ingredients (food) Nutrient Supplements
Nutrient Supplements Emulsifiers
Emulsifiers
 Collectors
Collectors Dust Suppressants
Dust Suppressants Explosives and Blasting Agents
Explosives and Blasting Agents Flocculants and Coagulants
Flocculants and Coagulants Frothers
Frothers Leaching Agents
Leaching Agents pH Modifiers
pH Modifiers Precious Metal Extraction Agents
Precious Metal Extraction Agents
 Antioxidants(plastic)
Antioxidants(plastic) Colorants (Pigments, Dyes)
Colorants (Pigments, Dyes) Fillers and Reinforcements
Fillers and Reinforcements Flame Retardants
Flame Retardants Monomers
Monomers Plasticizers
Plasticizers Polymerization Initiators
Polymerization Initiators Stabilizers (UV, Heat)
Stabilizers (UV, Heat)
 Antifoaming Agents
Antifoaming Agents Chelating Agents
Chelating Agents Coagulants and Flocculants
Coagulants and Flocculants Corrosion Inhibitors
Corrosion Inhibitors Disinfectants and Biocides
Disinfectants and Biocides Oxidizing Agents
Oxidizing Agents pH Adjusters
pH Adjusters Scale Inhibitors( water)
Scale Inhibitors( water)
 Antioxidants(cosmetic)
Antioxidants(cosmetic) Emollients
Emollients Fragrances and Essential Oils
Fragrances and Essential Oils Humectants
Humectants Preservatives
Preservatives Surfactants(cosmetic)
Surfactants(cosmetic) Thickeners
Thickeners UV Filters
UV Filters
 Fertilizers
Fertilizers Soil Conditioners
Soil Conditioners Plant Growth Regulators
Plant Growth Regulators Animal Feed Additives
Animal Feed Additives Biostimulants
Biostimulants Pesticides (Herbicides, Insecticides, Fungicides)
Pesticides (Herbicides, Insecticides, Fungicides)
 Active Pharmaceutical Ingredients (APIs)
Active Pharmaceutical Ingredients (APIs) Excipients
Excipients Solvents(pharmaceutical)
Solvents(pharmaceutical) Antibiotics
Antibiotics Antiseptics and Disinfectants
Antiseptics and Disinfectants Vaccine Adjuvants
Vaccine Adjuvants Nutraceutical Ingredients (pharmaceutical)
Nutraceutical Ingredients (pharmaceutical) Analgesics & Antipyretics
Analgesics & Antipyretics
 Analytical Reagents
Analytical Reagents Solvents(lab)
Solvents(lab) Chromatography Chemicals
Chromatography Chemicals Spectroscopy Reagents
Spectroscopy Reagents microbiology-and-cell-culture-reagents
microbiology-and-cell-culture-reagents Molecular Biology Reagents
Molecular Biology Reagents Biochemical Reagents
Biochemical Reagents Inorganic and Organic Standards
Inorganic and Organic Standards Laboratory Safety Chemicals
Laboratory Safety Chemicals Specialty Laboratory Chemicals(Special Laboratory Equipment)
Specialty Laboratory Chemicals(Special Laboratory Equipment)
 Demulsifiers
Demulsifiers Hydraulic Fracturing Fluids
Hydraulic Fracturing Fluids Scale Inhibitors(oil)
Scale Inhibitors(oil) Surfactants(oil)
Surfactants(oil) Drilling Fluids
Drilling Fluids
 Dyes and Pigments
Dyes and Pigments Bleaching Agents
Bleaching Agents Softening Agents
Softening Agents Finishing Agents
Finishing Agents Antistatic Agents
Antistatic Agents
 Admixtures
Admixtures Waterproofing Agents
Waterproofing Agents Sealants and Adhesives
Sealants and Adhesives Curing Compounds
Curing Compounds Concrete Repair Chemicals
Concrete Repair Chemicals Anti-Corrosion Coatings
Anti-Corrosion Coatings
 Surfactants(cleaning)
Surfactants(cleaning) Builders
Builders Enzymes
Enzymes Solvents (Cleaning)
Solvents (Cleaning) Fragrances
Fragrances
 Electronic Chemicals
Electronic Chemicals Catalysts
Catalysts Lubricants
Lubricants Photographic Chemicals
Photographic Chemicals Refrigerants
Refrigerants Automotive chemicals
Automotive chemicals Pyrotechnic Chemicals
Pyrotechnic Chemicals
 Biodegradable Surfactants
Biodegradable Surfactants Bio-based Solvents
Bio-based Solvents Renewable Polymers
Renewable Polymers Carbon Capture Chemicals
Carbon Capture Chemicals Wastewater Treatment Chemicals
Wastewater Treatment Chemicals
 Pigments
Pigments Solvents(paint)
Solvents(paint) Specialty Coatings
Specialty Coatings Binders/Resins
Binders/Resins Additives
Additives Driers
Driers Anti-Corrosion Agents
Anti-Corrosion Agents Functional Coatings
Functional Coatings Application-Specific Coatings
Application-Specific Coatings
 Fresh Herbs
Fresh Herbs Ground Spices
Ground Spices Whole Spices
Whole Spices Spice Blends
Spice Blends Dried Herbs
Dried Herbs
 Leavening Agents
Leavening Agents Dough Conditioners
Dough Conditioners Flour Treatments
Flour Treatments Fat Replacers
Fat Replacers Decoratives
Decoratives Preservatives(baking)
Preservatives(baking)
 Plasticizers & Softeners
Plasticizers & Softeners Reinforcing Agents
Reinforcing Agents Adhesion Promoters
Adhesion Promoters Vulcanizing Agents
Vulcanizing Agents Antidegradants
Antidegradants Blowing Agents
Blowing Agents Fillers & Extenders
Fillers & Extenders Accelerators & Retarders
Accelerators & Retarders
























































![Nickel Sulphate [NiSO4(H2O)6] Extra Pure Amaris Chemicals](https://amarischemicalsolutions.com/wp-content/uploads/2025/08/Nickel-Sulphate-NiSO4H2O6-Extra-Pure-Amaris-Chemicals-430x430.png)